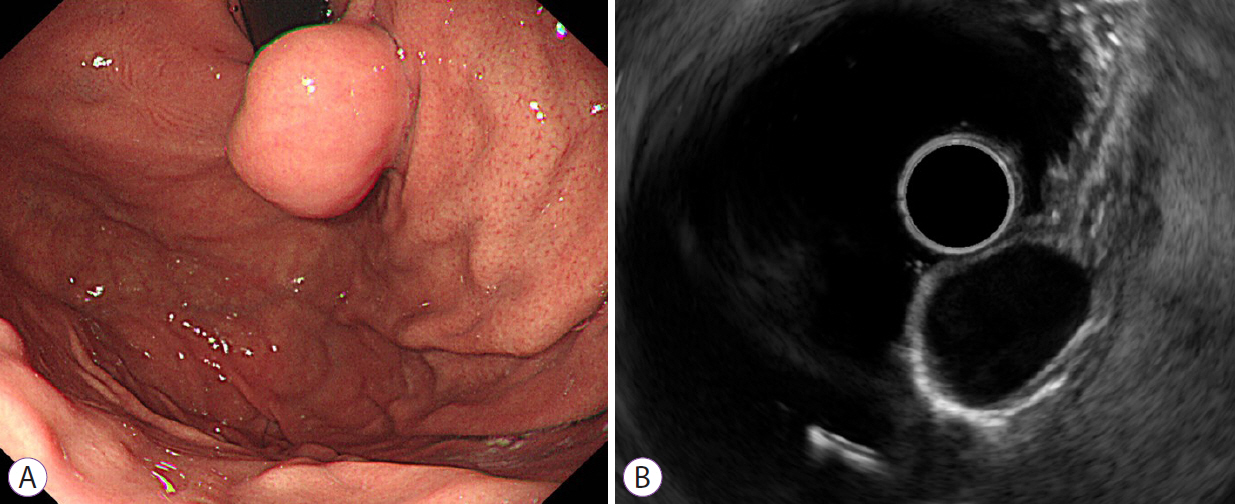
Clin Endosc.
2021 Nov;54(6):872-880. 10.5946/ce.2021.251.
Predictive Factors for Differentiating Gastrointestinal Stromal Tumors from Leiomyomas Based on Endoscopic Ultrasonography Findings in Patients with Gastric Subepithelial Tumors: A Multicenter Retrospective Study
- Affiliations
-
- 1Department of Internal Medicine, Konyang University College of Medicine, Daejeon, Korea
- 2Department of Internal Medicine, Daegu Catholic University School of Medicine, Daegu, Korea
- 3Department of Internal Medicine, Presbyterian Medical Center, Jeonju, Korea
- 4Department of Internal Medicine, Kyungpook National University Chilgok Hospital, Daegu, Korea
- 5Department of Internal Medicine, Inje University College of Medicine, Busan, Korea
- 6Department of Internal Medicine, Wonkwang University College of Medicine and Hospital, Iksan, Korea
- 7Department of Internal Medicine, Inje University College of Medicine, Seoul, Korea
- 8Department of Internal Medicine, Soonchunhyang University College of Medicine, Seoul, Korea
- KMID: 2522708
- DOI: http://doi.org/10.5946/ce.2021.251
Abstract
- Background/Aims
The utility of endoscopic ultrasonography (EUS) for differentiating gastrointestinal stromal tumors (GISTs) and leiomyomas of the stomach is not well known. We aimed to evaluate the ability of EUS for differentiating gastric GISTs and leiomyomas.
Methods
We retrospectively reviewed the medical records of patients with histopathologically proven GISTs (n=274) and leiomyomas (n=87). In two consensus meetings, the inter-observer variability in the EUS image analysis was reduced. Using logistic regression analyses, we selected predictive factors and constructed a predictive model and nomogram for differentiating GISTs from leiomyomas. A receiver operating characteristic (ROC) curve analysis was performed to measure the discrimination performance in the development and internal validation sets.
Results
Multivariate analysis identified heterogeneity (odds ratio [OR], 9.48), non-cardia (OR, 19.11), and older age (OR, 1.06) as independent predictors of GISTs. The areas under the ROC curve of the predictive model using age, sex, and four EUS factors (homogeneity, location, anechoic spaces, and dimpling or ulcer) were 0.916 (sensitivity, 0.908; specificity, 0.793) and 0.904 (sensitivity, 0.908; specificity, 0.782) in the development and internal validation sets, respectively.
Conclusions
The predictive model and nomogram using age, sex and homogeneity, tumor location, presence of anechoic spaces, and presence of dimpling or ulcer on EUS may facilitate differentiation between GISTs and leiomyomas.
Figure
Cited by 1 articles
-
Prevalence, natural progression, and clinical practices of upper gastrointestinal subepithelial lesions in Korea: a multicenter study
Younghee Choe, Yu Kyung Cho, Gwang Ha Kim, Jun-Ho Choi, Eun Soo Kim, Ji Hyun Kim, Eun Kwang Choi, Tae Hyeon Kim, Seong-Hun Kim, Do Hoon Kim
Clin Endosc. 2023;56(6):744-753. doi: 10.5946/ce.2023.005.
Reference
-
1. Lee JH, Lee HL, Ahn YW, et al. Prevalence of gastric subepithelial tumors in Korea: a single center experience. Korean J Gastroenterol. 2015; 66:274–276.
Article2. Lim YJ, Son HJ, Lee JS, et al. Clinical course of subepithelial lesions detected on upper gastrointestinal endoscopy. World J Gastroenterol. 2010; 16:439–444.
Article3. Hwang JH, Rulyak SD, Kimmey MB; American Gastroenterological Association Institute. American Gastroenterological Association Institute technical review on the management of gastric subepithelial masses. Gastroenterology. 2006; 130:2217–2228.
Article4. Cho JW; Korean ESD Study Group. Current guidelines in the management of upper gastrointestinal subepithelial tumors. Clin Endosc. 2016; 49:235–240.
Article5. Menon L, Buscaglia JM. Endoscopic approach to subepithelial lesions. Therap Adv Gastroenterol. 2014; 7:123–130.
Article6. Miettinen M, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. Am J Surg Pathol. 2005; 29:52–68.7. Agaimy A. Gastrointestinal stromal tumors (GIST) from risk stratification systems to the new TNM proposal: more questions than answers? A review emphasizing the need for a standardized GIST reporting. Int J Clin Exp Pathol. 2010; 3:461–471.8. Kim GH, Park DY, Kim S, et al. Is it possible to differentiate gastric GISTs from gastric leiomyomas by EUS? World J Gastroenterol. 2009; 15:3376–3381.
Article9. Seo SW, Hong SJ, Han JP, et al. Accuracy of a scoring system for the differential diagnosis of common gastric subepithelial tumors based on endoscopic ultrasonography. J Dig Dis. 2013; 14:647–653.
Article10. ASGE Standards of Practice Committee, Gan SI, Rajan E, et al. Role of EUS. Gastrointest Endosc. 2007; 66:425–434.
Article11. He G, Wang J, Chen B, et al. Feasibility of endoscopic submucosal dissection for upper gastrointestinal submucosal tumors treatment and value of endoscopic ultrasonography in pre-operation assess and post-operation follow-up: a prospective study of 224 cases in a single medical center. Surg Endosc. 2016; 30:4206–4213.
Article12. Gress F, Schmitt C, Savides T, et al. Interobserver agreement for EUS in the evaluation and diagnosis of submucosal masses. Gastrointest Endosc. 2001; 53:71–76.
Article13. Lee HH, Hur H, Jung H, Jeon HM, Park CH, Song KY. Analysis of 151 consecutive gastric submucosal tumors according to tumor location. J Surg Oncol. 2011; 104:72–75.
Article14. Lee JS, Kim JJ, Park SM. Laparoscopic gastric wedge resection and prophylactic antireflux surgery for a submucosal tumor of gastroesophageal junction. J Gastric Cancer. 2011; 11:131–134.
Article15. Kim GH, Ahn JY, Gong CS, et al. Efficacy of endoscopic ultrasound-guided fine-needle biopsy in gastric subepithelial tumors located in the cardia. Dig Dis Sci. 2020; 65:583–590.
Article16. Min YW, Park HN, Min BH, Choi D, Kim KM, Kim S. Preoperative predictive factors for gastrointestinal stromal tumors: analysis of 375 surgically resected gastric subepithelial tumors. J Gastrointest Surg. 2015; 19:631–638.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Incidental Gastrointestinal Subepithelial Mass
- Endoscopic Ultrasonography in the Diagnosis of Gastric Subepithelial Lesions
- Long-Term Outcomes after Endoscopic Treatment of Gastric Gastrointestinal Stromal Tumor
- Contrast Enhanced Endoscopic Ultrasound Imaging for Gastrointestinal Subepithelial Tumors
- Common Gastric Subepithelial Tumors in Koreans