J Korean Med Sci.
2021 Nov;36(45):e318. 10.3346/jkms.2021.36.e318.
Safety Monitoring after the BNT162b2 COVID-19 Vaccine among Adults Aged 75 Years or Older
- Affiliations
-
- 1Department of Pediatrics, National Medical Center, Seoul, Korea
- 2Department of Infectious Diseases, National Medical Center, Seoul, Korea
- 3Department of Gastroenterology and Hepatology, National Medical Center, Seoul, Korea
- 4Department of Anesthesiology and Pain Medicine, National Medical Center, Seoul, Korea
- KMID: 2522555
- DOI: http://doi.org/10.3346/jkms.2021.36.e318
Abstract
- Background
Older adults are given high priority for coronavirus disease 2019 (COVID-19) vaccination; however, little is known about the safety of vaccines. This study was conducted to examine the safety of the COVID-19 vaccine for people who were ≥ 75 years of age, specifically those who first took two doses of the vaccine at the COVID-19 central vaccination center in South Korea.
Methods
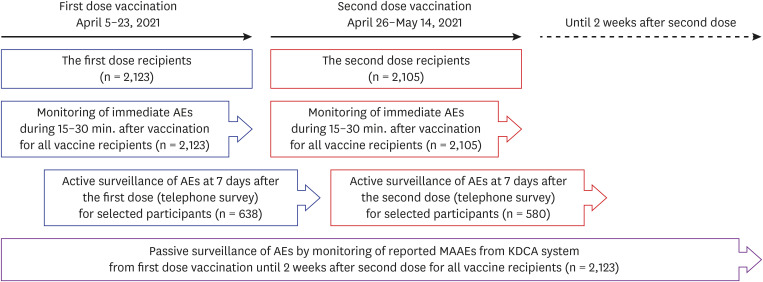
Safety monitoring after the BNT162b2 vaccine was conducted in three ways for older adults who received the first dose of the vaccine at our center between April 5 and April 23, 2021. For immediate adverse reactions, every person who was vaccinated was observed for 15–30 minutes after injection at the center. For active surveillance, a telephone interview was conducted for stratified randomly sampled people after 7 days of each vaccination to enquire regarding types of adverse reactions they experienced, and its severity and duration. For passive surveillance, reported adverse event data were collected from the COVID-19 vaccine adverse event following immunization (AEFI) surveillance system—run by the Korea Disease Control and Prevention Agency (KDCA). The data were then reviewed.
Results
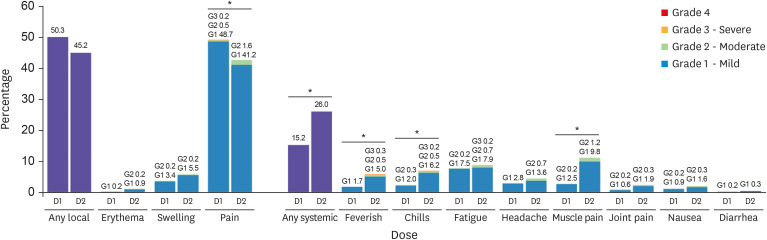
In total, 2,123 older adults received at least one vaccine dose during the study period. The frequency of acute adverse reactions that developed during the observed 15–30 minutes after injection was 8.5 cases per 1,000 doses. None of the reactions was assessed as acute allergic reactions to the vaccine and no cases required special treatment or drug administration. Overall, 638 people were followed up at least once by telephone interview 7 days post vaccination. The overall response rate was 82.3%. The rates of local reactions were 50.3% after the first dose and 45.2% after the second dose, and the rates of systemic reactions were 15.2% and 26.0%, respectively. During the study period, 23 medically attended adverse events (5.4 cases per 1,000 administered doses) were reported to the KDCA AEFI surveillance system. The most common symptoms of medically attended cases were nonspecific general weakness (26%) and dizziness (26%), followed by muscle pain (22%), headache (13%), fever (13%), and skin rash or urticaria (13%). Among them, there were five serious adverse events reported, which required hospitalization, including one death. However, most of them were not related to the vaccines.
Conclusion
BNT162b2 vaccination was tolerable among adults who were ≥ 75 years of age.
Keyword
Figure
Cited by 2 articles
-
A Nationwide Survey of mRNA COVID-19 Vaccinee’s Experiences on Adverse Events and Its Associated Factors
Dongwon Yoon, Ha-Lim Jeon, Yunha Noh, Young June Choe, Seung-Ah Choe, Jaehun Jung, Ju-Young Shin
J Korean Med Sci. 2023;38(22):e170. doi: 10.3346/jkms.2023.38.e170.Effective Vaccination and Education Strategies for Emerging Infectious Diseases Such as COVID-19
Seong-Heon Wie, Jaehun Jung, Woo Joo Kim
J Korean Med Sci. 2023;38(44):e371. doi: 10.3346/jkms.2023.38.e371.
Reference
-
1. World Health Organization. WHO coronavirus (COVID-19) dashboard. Updated 2021. Accessed July 13, 2021. https://covid19.who.int/ .2. Korea Disease Control and Prevention Agency. Press release. COVID-19 vaccination to begin this week. Updated February 22, 2021. Accessed July 13, 2021. https://www.kdca.go.kr/board/board.es?mid=a30402000000&bid=0030 .3. Du RH, Liang LR, Yang CQ, Wang W, Cao TZ, Li M, et al. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: a prospective cohort study. Eur Respir J. 2020; 55(5):2000524. PMID: 32269088.
Article4. Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020; 180(7):934–943. PMID: 32167524.
Article5. Soiza RL, Scicluna C, Thomson EC. Efficacy and safety of COVID-19 vaccines in older people. Age Ageing. 2021; 50(2):279–283. PMID: 33320183.
Article6. Choi MJ, Choi WS, Seong H, Choi JY, Kim JH, Kim YJ, et al. Developing a framework for pandemic COVID-19 vaccine allocation: a modified Delphi consensus study in Korea. J Korean Med Sci. 2021; 36(23):e166. PMID: 34128597.
Article7. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020; 383(27):2603–2615. PMID: 33301246.
Article8. Walsh EE, Frenck RW Jr, Falsey AR, Kitchin N, Absalon J, Gurtman A, et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med. 2020; 383(25):2439–2450. PMID: 33053279.
Article9. Bae S, Lee YW, Lim SY, Lee JH, Lim JS, Lee S, et al. Adverse reactions following the first dose of ChAdOx1 nCoV-19 vaccine and BNT162b2 vaccine for healthcare workers in South Korea. J Korean Med Sci. 2021; 36(17):e115. PMID: 33942579.
Article10. Lee YW, Lim SY, Lee JH, Lim JS, Kim M, Kwon S, et al. Adverse reactions of the second dose of the BNT162b2 mRNA COVID-19 vaccine in healthcare workers in Korea. J Korean Med Sci. 2021; 36(21):e153. PMID: 34060261.
Article11. Song JY, Cheong HJ, Kim SR, Lee SE, Kim SH, Noh JY, et al. Early safety monitoring of COVID-19 vaccines in healthcare workers. J Korean Med Sci. 2021; 36(15):e110. PMID: 33876589.
Article12. Food and Drug Administration. Vaccines and related biological products Advisory Committee Meeting December 10, 2020, FDA briefing document, Pfizer-BioNTech COVID-19 Vaccine. Updated 2020. Accessed July 13, 2021. https://www.fda.gov/media/144245/download .13. Menni C, Klaser K, May A, Polidori L, Capdevila J, Louca P, et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID symptom study app in the UK: a prospective observational study. Lancet Infect Dis. 2021; 21(7):939–949. PMID: 33930320.
Article14. Müller L, Andrée M, Moskorz W, Drexler I, Walotka L, Grothmann R, et al. Age-dependent immune response to the Biontech/Pfizer BNT162b2 COVID-19 vaccination. Clin Infect Dis. Forthcoming. 2021; DOI: 10.1093/cid/ciab381.
Article15. Kim SH, Wi YM, Yun SY, Ryu JS, Shin JM, Lee EH, et al. Adverse events in healthcare workers after the first dose of ChAdOx1 nCoV-19 or BNT162b2 mRNA COVID-19 vaccination: a single center experience. J Korean Med Sci. 2021; 36(14):e107. PMID: 33847085.
Article16. Klein SL, Jedlicka A, Pekosz A. The Xs and Y of immune responses to viral vaccines. Lancet Infect Dis. 2010; 10(5):338–349. PMID: 20417416.
Article17. Harris T, Nair J, Fediurek J, Deeks SL. Assessment of sex-specific differences in adverse events following immunization reporting in Ontario, 2012-15. Vaccine. 2017; 35(19):2600–2604. PMID: 28365252.
Article18. Klein SL, Marriott I, Fish EN. Sex-based differences in immune function and responses to vaccination. Trans R Soc Trop Med Hyg. 2015; 109(1):9–15. PMID: 25573105.
Article19. Decaroli MC, Rochira V. Aging and sex hormones in males. Virulence. 2017; 8(5):545–570. PMID: 27831823.
Article20. Gee J, Marquez P, Su J, Calvert GM, Liu R, Myers T, et al. First month of COVID-19 vaccine safety monitoring - United States, December 14, 2020-January 13, 2021. MMWR Morb Mortal Wkly Rep. 2021; 70(8):283–288. PMID: 33630816.
Article21. Oh HK, Lee YK, Kim TE, Lee EJ, Park SK. Status of adverse reactions after COVID-19 vaccination in the Republic of Korea, February 26, 2021-March 25, 2021. Updated April 29, 2021. Accessed July 13, 2021. http://kdca.go.kr/board/board.es?mid=a20602010000&bid=0034&list_no=713170&act=view .22. Center for Biologics Evaluation and Research Office of Biostatistics and Epidemiology. CBER surveillance program background rates of adverse events of special interest for COVID-19 vaccine safety monitoring protocol. Updated 2021. Accessed July 13, 2021. https://www.bestinitiative.org/wp-content/uploads/2021/02/C19-Vaccine-Safety-AESI-Background-Rate-Protocol-FINAL-2020.pdf .23. Ozonoff A, Nanishi E, Levy O. Bell's palsy and SARS-CoV-2 vaccines. Lancet Infect Dis. 2021; 21(4):450–452. PMID: 33639103.
Article24. Shemer A, Pras E, Einan-Lifshitz A, Dubinsky-Pertzov B, Hecht I. Association of COVID-19 vaccination and facial nerve palsy: a case-control study. JAMA Otolaryngol Head Neck Surg. 2021; 147(8):739–743. PMID: 34165512.25. European Medicines Agency. COVID-19 vaccine safety update for Comirnaty: 18 June 2021. Updated 2021. Accessed July 13, 2021. https://www.ema.europa.eu/en/documents/covid-19-vaccine-safety-update/covid-19-vaccine-safety-update-comirnaty-18-june-2021_en.pdf .26. European Medicines Agency. Comirnaty: EPAR-product information. Updated 2021. Accessed July 16, 2021. https://www.ema.europa.eu/en/documents/product-information/comirnaty-epar-product-information_en.pdf .27. Alroy-Preis S, Milo R. Booster protection against confirmed infections and severe disease - data from Israel. Updated September 17, 2021. Accessed October 6, 2021. https://www.fda.gov/media/152205/download .
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Review of COVID-19 Vaccines and Their Evidence in Older Adults
- Efficacy and Safety of COVID-19 Vaccines in Children Aged 5 to 11 Years: A Systematic Review
- A Case of Leukocytoclastic Vasculitis Following COVID-19 Vaccination
- Adult-onset Still’s Disease after BNT162b2 mRNA COVID-19 Vaccine
- Self-reported adverse events after 2 doses of COVID-19 vaccine in Korea