J Korean Neurosurg Soc.
2021 Nov;64(6):864-872. 10.3340/jkns.2021.0032.
Synthesis of New Boron Derived Compounds; Anticancer, Antioxidant and Antimicrobial Effect in Vitro Glioblastoma Tumor Model
- Affiliations
-
- 1Training and Research Center, Kutahya Health Sciences University, Kütahya, Turkey
- 2Department of Neurosurgery, Kutahya Health Sciences University, Kütahya, Turkey
- 3Department of Chemistry, Hitit University, Çorum, Turkey
- 4Department of Neurosurgery, Eskişehir Osmangazi University, Eskisehir, Turkey
- KMID: 2521976
- DOI: http://doi.org/10.3340/jkns.2021.0032
Abstract
Objective
: The aim of our study is to investigate the cytotoxic, antioxidant, and antimicrobial effects of newly synthesized boron compounds in U87MG glioblastoma cell treatment.
Methods
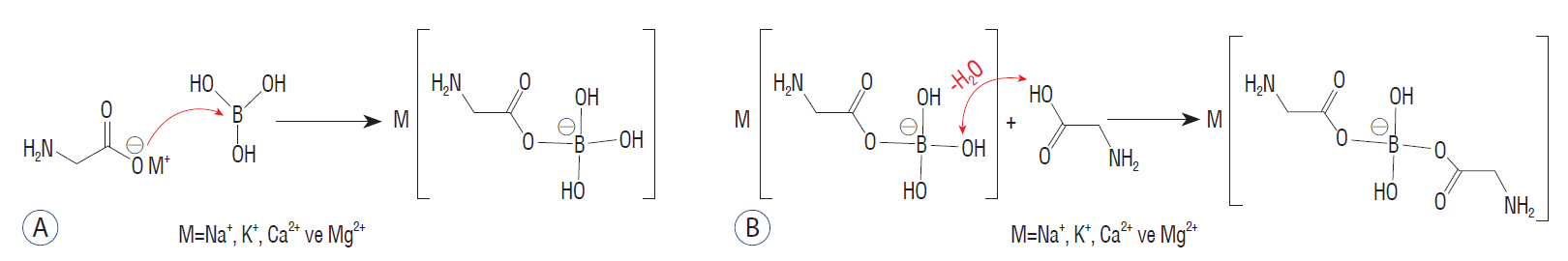
: We synthesized boron glycine monoester (BGM) and boron glycine diester (BGD) structures containing boron atoms and determined their cytotoxic activities on glioblastoma by the MTT method. The IC50 value was calculated with GraphPad Prism 5.0 program. The IC50 values were administered 48 hours on U87MG glioblastoma cell. Catalase (CAT), acid phosphatase (ACP) and alkaline phosphatase (ALP) enzyme activity, malondialdehyde (MDA), total glutathione (GSH), and total protein levels were detected using spectrophotometric methods. We determined the antimicrobial activities of BGM and BGD with the disc diffusion method.
Results
: After 48 hours of BGM and BGD application to U87MG glioblastoma cells, we found the IC50 value as 6.6 mM and 26 mM, respectively. CAT and ACP enzyme activities were decreased in BGM and BGD groups. MDA which is a metabolite of lipid peroxidation was increased in both boron compounds groups. GSH level was reduced especially in BGD group. BGM and BGD have been found to be antimicrobial effects.
Conclusion
: Boron compounds, especially the BGM, can provide a new therapeutic approach for the treatment of glioblastoma with their anticancer, antioxidant, and antimicrobial effects.
Keyword
Figure
Reference
-
References
1. Aebi H. Catalase in vitro. In : Bergmeyer HU, editor. Methods of Enzymatic Analysis. ed 2. London: Academic Press;1974. p. 121–126.2. Ahmad S, Haque MM, Ashraf SM, Ahmad S. Urethane modified boron filled polyesteramide: a novel anti-microbial polymer from a sustainable resource. Eur Polym J. 40:2097–2104. 2004.
Article3. Ali F, Hosmane NS, Zhu Y. Boron chemistry for medical applications. Molecules. 25:828. 2020.
Article4. Axtell JC, Saleh LMA, Qian EA, Wixtrom AI, Spokoyny AM. Synthesis and applications of perfunctionalized boron clusters. Inorg Chem. 57:2333–2350. 2018.
Article5. Aydin HE, Koldemir-Gündüz M, Kizmazoglu C, Kandemir T, Arslantas A. Cytotoxic effect of boron application on glioblastoma cells. Turk Neurosurg. 31:206–210. 2021.
Article6. Backos DS, Franklin CC, Reigan P. The role of glutathione in brain tumor drug resistance. Biochem Pharmacol. 83:1005–1012. 2012.
Article7. Barth RF, Zhang Z, Liu T. A realistic appraisal of boron neutron capture therapy as a cancer treatment modality. Cancer Commun (Lond). 38:36. 2018.
Article8. Beutler E. Glutathione in Red Cell Metabolism: A Manual of Biochemical Methods. ed 2. New York: Grune & Stratton;1975. p. 112–114.9. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 72:248–254. 1976.
Article10. Canturk Z, Tunali Y, Korkmaz S, Gulbaş Z. Cytotoxic and apoptotic effects of boron compounds on leukemia cell line. Cytotechnology. 68:87–93. 2016.
Article11. Çelik B, Ersöz E, Korkmaz M. Investigation of the therapy potential of borax pentahydrate in glioblastoma multiforme cell line. J Boron. 5:56–61. 2020.12. Çelik SY, Demir N, Demir Y. The in-vitro effect of lisonopril on serum alkaline phosphatase and acid phosphatase enzymes activity. CBU J Sci. 13:233–237. 2017.13. Çiftçi N. The role of oxidative stress in cancer: could antioxidants fuel the progression of cancer? Ahi Evran Tıp Dergisi. 1:8–13. 2017.14. Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial disk susceptibility tests; approved standard, eleventh edition. Wayne: CLSI;2012.15. Fojo T, Bates S. Strategies for reversing drug resistance. Oncogene. 22:7512–7523. 2003.
Article16. Gündüz MK, Bolat M, Kaymak G, Berikten D, Köse DA. Therapeutic effects of newly synthesized boron compounds (BGM and BGD) on hepatocellular carcinoma. Biol Trace Elem Res. 2021; [Epub ahead of print].
Article17. Halliwell B. Oxidative stress and neurodegeneration: where are we now? J Neurochem. 97:1634–1658. 2006.
Article18. Hawthorne MF. The role of chemistry in the development of boron neutron capture therapy of cancer. Angew Chem Int Ed Engl. 32:950–984. 1993.
Article19. Hawthorne MF, Maderna A. Applications of radiolabeled boron clusters to the diagnosis and treatment of cancer. Chem Rev. 99:3421–3434. 1999.
Article20. Kachadourian R, Brechbuhl HM, Ruiz-Azuara L, Gracia-Mora I, Day BJ. Casiopeína IIgly-induced oxidative stress and mitochondrial dysfunction in human lung cancer A549 and H157 cells. Toxicology. 268:176–183. 2010.
Article21. Kaynar H, Meral M, Turhan H, Keles M, Celik G, Akcay F. Glutathione peroxidase, glutathione-S-transferase, catalase, xanthine oxidase, CuZn superoxide dismutase activities, total glutathione, nitric oxide, and malondialdehyde levels in erythrocytes of patients with small cell and non-small cell lung cancer. Cancer Lett. 227:133–139. 2005.
Article22. Köse DA. Preparation and structure investigation of biopotent boron compounds with hydroxy-functionalized organic molecules. Ankara: Hacettepe University, Science Institute;2008.23. Köse DA, Karan Zümreoğlu B, Hökelek T, Şahin E. Boric acid complexes with organic biomolecules: mono-chelate complexes with salicylic and glucuronic acids. Inorganica Chimica Acta. 363:4031–4037. 2010.
Article24. Köse DA, Zümreoglu-Karan B. Mixed ligand complexes of boric acid with organic biomolecules. Chemical Papers. 66:54–60. 2012.25. Köse DA, Zumreoglu-Karan B, Hökelek T. A comparative examination of mono- and Bis-chelate salicylatoborate complexes and the crystal structure of layered magnesium Bis-salicylatoborate. Inorganica Chimica Acta. 375:236–241. 2011.
Article26. Ledwozyw A, Michalak J, Stepień A, Kadziołka A. The relationship between plasma triglycerides, cholesterol, total lipids and lipid peroxidation products during human atherosclerosis. Clin Chim Acta. 155:275–283. 1986.
Article27. Meiyanto E, Susidarti RA, Jenie RI, Utomo RY, Novitasari D, Wulandari F, et al. Synthesis of new boron containing compound (CCB-2) based on curcumin structure and its cytotoxic effect against cancer cells. J Appl Pharm Sci. 10:060–066. 2020.
Article28. Ostrom QT, Bauchet L, Davis FG, Deltour I, Fisher JL, Langer CE, et al. The epidemiology of glioma in adults: a “state of the science” review. Neuro Oncol. 16:896–913. 2014.
Article29. Pointer BR, Boyer MP, Schmidt M. Boric acid destabilizes the hyphal cytoskeleton and inhibits invasive growth of Candida albicans. Yeast. 32:389–398. 2015.
Article30. Qian EA, Wixtrom AI, Axtell JC, Saebi A, Jung D, Rehak P, et al. Atomically precise organomimetic cluster nanomolecules assembled via perfluoroaryl-thiol SNAr chemistry. Nat Chem. 9:333–340. 2017.
Article31. Rajneesh CP, Manimaran A, Sasikala KR, Adaikappan P. Lipid peroxidation and antioxidant status in patients with breast cancer. Singapore Med J. 49:640–643. 2008.32. Ray G, Batra S, Shukla NK, Deo S, Raina V, Ashok S, et al. Lipid peroxidation, free radical production and antioxidant status in breast cancer. Breast Cancer Res Treat. 59:163–170. 2000.
Article33. Rock K, McArdle O, Forde P, Dunne M, Fitzpatrick D, O’Neill B, et al. A clinical review of treatment outcomes in glioblastoma multiforme--the validation in a non-trial population of the results of a randomised phase III clinical trial: has a more radical approach improved survival? Br J Radiol. 85:e729–e733. 2012.
Article34. Sarac N, Ugur A, Boran R, Elgin ES. The use of boron compounds for stabilization of lipase from pseudomonas aeruginosa ES3 for the detergent industry. J Surfactants Deterg. 18:275–285. 2015.
Article35. Sarı P. Investigation of the biochemical effects of epigallocatechin gallate and resveratrol in cultured C6 glioma cells. Sağlık Bilimleri Enstitüsü: Istanbul Science University;2011.36. Sayin Z, Ucan US, Sakmanoglu A. Antibacterial and antibiofilm effects of boron on different bacteria. Biol Trace Elem Res. 173:241–246. 2016.
Article37. Schumacker PT. Reactive oxygen species in cancer: a dance with the devil. Cancer Cell. 27:156–157. 2015.
Article38. Uysal N, Gönenç S, SönmeZ A, Aksu İ, Topçu A, Kayatekin BM, et al. Antioxidant enzyme activities and lipid peroxidation levels in adolescent rat brain. Ege Tıp Dergisi. 44:75–79. 2005.39. Walter K, Schült C. Acid and alkaline phosphatase in serum (two point method). In : Bergmeyer HU, editor. Methods of Enzymatic Analysis. ed 2. Cambridge: Academic Press;1974. 2:p. 856–886.40. Yerlikaya A, Okur E, Eker S, Erin N. Combined effects of the proteasome inhibitor bortezomib and Hsp70 inhibitors on the B16F10 melanoma cell line. Mol Med Rep. 3:333–339. 2010.
Article41. Yılmaz MT. Minimum inhibitory and minimum bactericidal concentrations of boron compounds against several bacterial strains. Turk J Med Sci. 42:1423–1429. 2012.42. Zabłocka-Słowińska K, Płaczkowska S, Skórska K, Prescha A, Pawełczyk K, Porębska I, et al. Oxidative stress in lung cancer patients is associated with altered serum markers of lipid metabolism. PLoS One. 14:e0215246. 2019.
Article43. Zanders ED, Svensson F, Bailey DS. Therapy for glioblastoma: is it working? Drug Discov Today. 24:1193–1201. 2019.
Article44. Zavjalov E, Zaboronok A, Kanygin V, Kasatova A, Kichigin A, Mukhamadiyarov R, et al. Accelerator-based boron neutron capture therapy for malignant glioma: a pilot neutron irradiation study using boron phenylalanine, sodium borocaptate and liposomal borocaptate with a heterotopic U87 glioblastoma model in SCID mice. Int J Radiat Biol. 96:868–878. 2020.
Article45. Zhu Y, Gao S, Hosmane NS. Boron-enriched advanced energy materials. Inorg Chim. 471:577–586. 2018.
Article46. Zhu Y, Hosmane NS. Ionic liquids: recent advances and applications in boron chemistry. Eur J Inorg Chem. 2017:4369–4377. 2017.
Article47. Zhu Y, Hosmane NS. Nanostructured boron compounds for cancer therapy. Pure Appl Chem. 90:653–663. 2018.
Article48. Zumreoglu-Karan B, Kose DA. Boric acid: a simple molecule of physiologic, therapeutic and prebiotic significance. Pure Appl Chem. 87:155–162. 2015.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Boron Supplementation on Lipid Profiles and Antioxidant Capacities in the Ovariectomized Rats
- Effect of Intralesional Interleukin-2 on Murine Bladder Tumor
- The Effect of Rhus verniciflua Stokes Extracts on Photo-Aged Mouse Skin
- Sex-dependent liver cancer xenograft models for predicting clinical data in the evaluation of anticancer drugs
- Exploring the Potential of Rosemary Derived Compounds (Rosmarinic and Carnosic Acids) as Cancer Therapeutics: Current Knowledge and Future Perspectives