Endocrinol Metab.
2021 Oct;36(5):1055-1068. 10.3803/EnM.2021.1104.
Musclin Is Related to Insulin Resistance and Body Composition, but Not to Body Mass Index or Cardiorespiratory Capacity in Adults
- Affiliations
-
- 1Physiology and Biochemistry Research Group-PHYSIS, Faculty of Medicine, University of Antioquia, Medellin, Colombia
- 2Indeportes Antioquia, Medellin, Colombia
- 3Pablo Tobón Uribe Hospital, Medellin, Colombia
- 4Sports Medicine Postgraduate Program, and GRINMADE Research Group, SICOR Center, Faculty of Medicine, University of Antioquia, Medellin, Colombia
- KMID: 2521953
- DOI: http://doi.org/10.3803/EnM.2021.1104
Abstract
- Background
We studied whether musclin function in humans is related to glycemic control, body composition, and cardiorespiratory capacity.
Methods
A cross-sectional study was performed in sedentary adults with or without metabolic syndrome (MS). Serum musclin was measured by enzyme-linked immunosorbent assay. Insulin resistance (IR) was evaluated by the homeostatic model assessment (HOMA-IR). Body composition was determined by dual-energy X-ray absorptiometry and muscle composition by measuring carnosine in the thigh, a surrogate of fiber types, through proton magnetic resonance spectroscopy. Cardiorespiratory capacity was assessed through direct ergospirometry.
Results
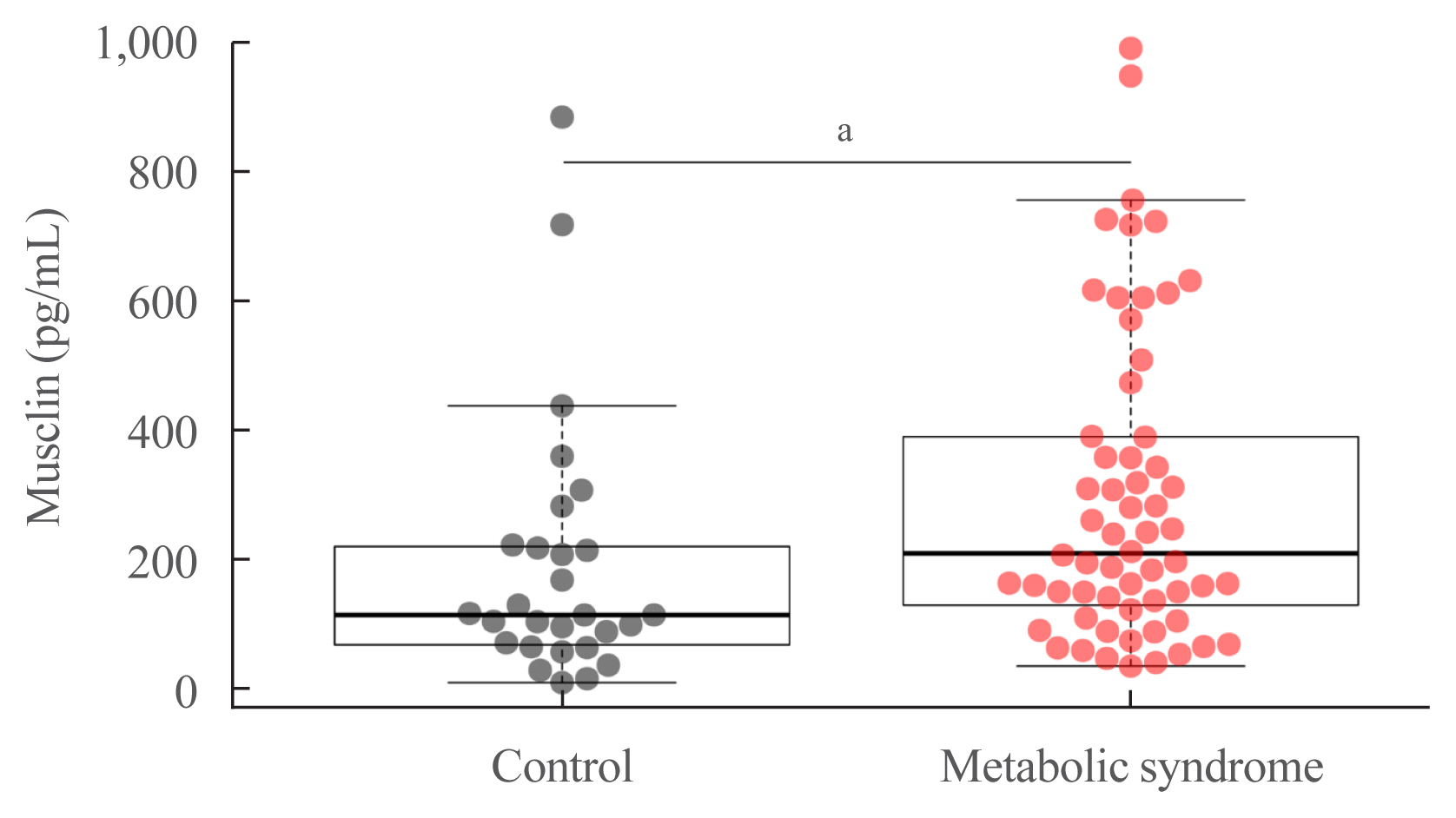
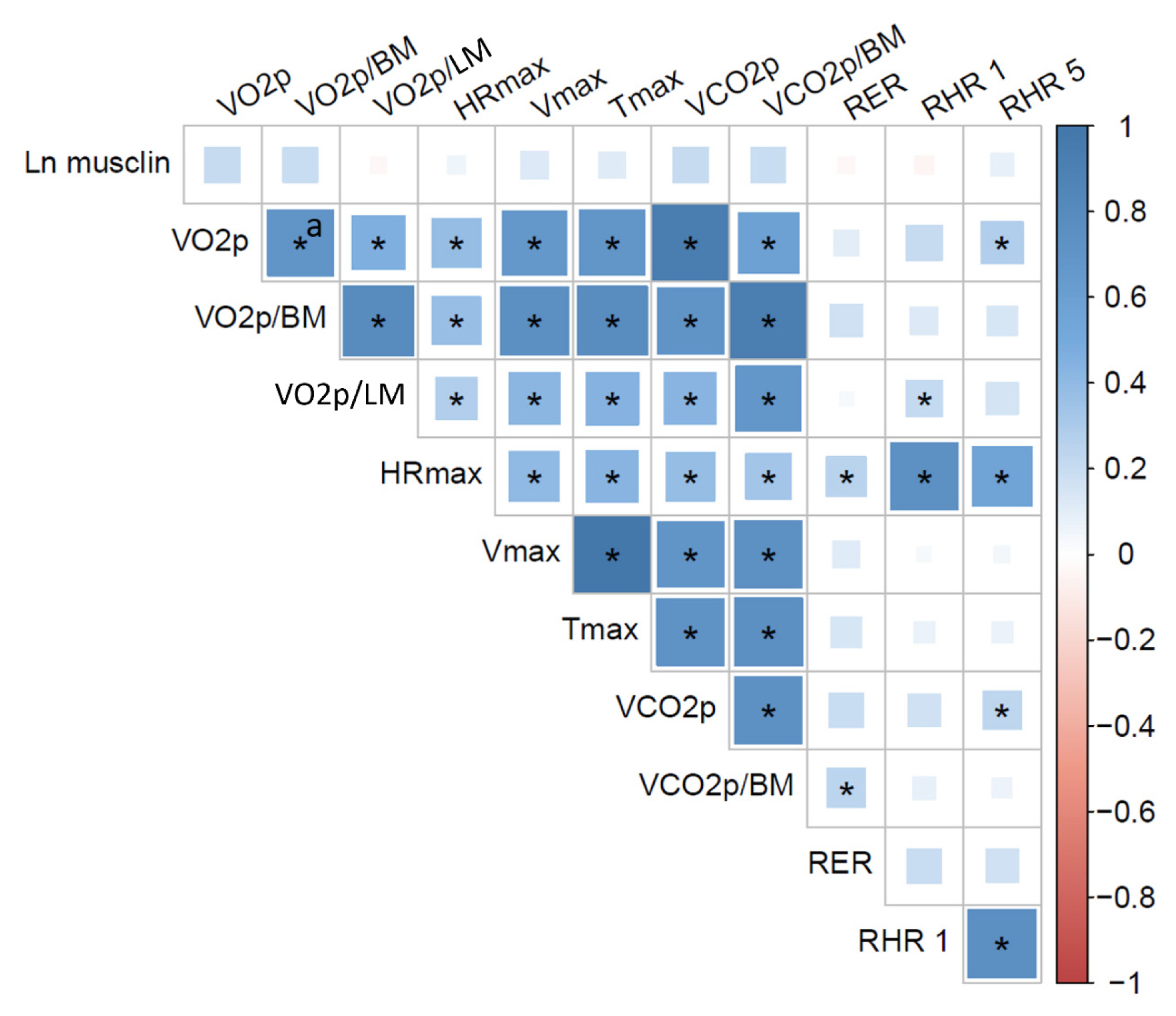
The control (n=29) and MS (n=61) groups were comparable in age (51.5±6.5 years old vs. 50.7±6.1 years old), sex (72.4% vs. 70.5% women), total lean mass (58.5%±7.4% vs. 57.3%±6.8%), and peak oxygen consumption (VOpeak) (31.0±5.8 mL O2./kg.min vs. 29.2±6.3 mL O2/kg.min). Individuals with MS had higher body mass index (BMI) (30.6±4.0 kg/m2 vs. 27.4± 3.6 kg/m2), HOMA-IR (3.5 [95% confidence interval, CI, 2.9 to 4.6] vs. 1.7 [95% CI, 1.1 to 2.0]), and musclin (206.7 pg/mL [95% CI, 122.7 to 387.8] vs. 111.1 pg/mL [95% CI, 63.2 to 218.5]) values than controls (P˂0.05). Musclin showed a significant relationship with HOMA-IR (β=0.23; 95% CI, 0.12 to 0.33; P˂0.01), but not with VOpeak, in multiple linear regression models adjusted for age, sex, fat mass, lean mass, and physical activity. Musclin was significantly associated with insulin, glycemia, visceral fat, and regional muscle mass, but not with BMI, VCO2peak, maximum heart rate, maximum time of work, or carnosine.
Conclusion
In humans, musclin positively correlates with insulinemia, IR, and a body composition profile with high visceral adiposity and lean mass, but low body fat percentage. Musclin is not related to BMI or cardiorespiratory capacity.
Keyword
Figure
Reference
-
1. DeFronzo RA. Lilly lecture 1987: the triumvirate: beta-cell, muscle, liver: a collusion responsible for NIDDM. Diabetes. 1988; 37:667–87.2. Lauritzen HP, Galbo H, Toyoda T, Goodyear LJ. Kinetics of contraction-induced GLUT4 translocation in skeletal muscle fibers from living mice. Diabetes. 2010; 59:2134–44.
Article3. Lund S, Holman GD, Schmitz O, Pedersen O. Contraction stimulates translocation of glucose transporter GLUT4 in skeletal muscle through a mechanism distinct from that of insulin. Proc Natl Acad Sci U S A. 1995; 92:5817–21.
Article4. Atlantis E, Martin SA, Haren MT, Taylor AW, Wittert GA. Members of the Florey Adelaide Male Ageing Study. Inverse associations between muscle mass, strength, and the metabolic syndrome. Metabolism. 2009; 58:1013–22.
Article5. Jurca R, Lamonte MJ, Barlow CE, Kampert JB, Church TS, Blair SN. Association of muscular strength with incidence of metabolic syndrome in men. Med Sci Sports Exerc. 2005; 37:1849–55.
Article6. Londono FJ, Calderon JC, Gallo J. Association between thigh muscle development and the metabolic syndrome in adults. Ann Nutr Metab. 2012; 61:41–6.
Article7. Park BS, Yoon JS. Relative skeletal muscle mass is associated with development of metabolic syndrome. Diabetes Metab J. 2013; 37:458–64.
Article8. Srikanthan P, Karlamangla AS. Relative muscle mass is inversely associated with insulin resistance and prediabetes: findings from the third National Health and Nutrition Examination Survey. J Clin Endocrinol Metab. 2011; 96:2898–903.
Article9. Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012; 481:463–8.
Article10. Liu Y, Huo X, Pang XF, Zong ZH, Meng X, Liu GL. Musclin inhibits insulin activation of Akt/protein kinase B in rat skeletal muscle. J Int Med Res. 2008; 36:496–504.
Article11. Nishizawa H, Matsuda M, Yamada Y, Kawai K, Suzuki E, Makishima M, et al. Musclin, a novel skeletal muscle-derived secretory factor. J Biol Chem. 2004; 279:19391–5.
Article12. Ahima RS, Park HK. Connecting myokines and metabolism. Endocrinol Metab (Seoul). 2015; 30:235–45.
Article13. Narvaez-Sanchez R, Calderon JC, Vega G, Trillos MC, Ospina S. Skeletal muscle as a protagonist in the pregnancy metabolic syndrome. Med Hypotheses. 2019; 126:26–37.
Article14. Kim G, Kim JH. Impact of skeletal muscle mass on metabolic health. Endocrinol Metab (Seoul). 2020; 35:1–6.
Article15. Chen WJ, Liu Y, Sui YB, Yang HT, Chang JR, Tang CS, et al. Positive association between musclin and insulin resistance in obesity: evidence of a human study and an animal experiment. Nutr Metab (Lond). 2017; 14:46.
Article16. Banzet S, Koulmann N, Sanchez H, Serrurier B, Peinnequin A, Bigard AX. Musclin gene expression is strongly related to fast-glycolytic phenotype. Biochem Biophys Res Commun. 2007; 353:713–8.
Article17. Tanner CJ, Barakat HA, Dohm GL, Pories WJ, MacDonald KG, Cunningham PR, et al. Muscle fiber type is associated with obesity and weight loss. Am J Physiol Endocrinol Metab. 2002; 282:E1191–6.18. Guo Q, Hu H, Liu X, Yang D, Yin Y, Zhang B, et al. C/EBPβ mediates palmitate-induced musclin expression via the regulation of PERK/ATF4 pathways in myotubes. Am J Physiol Endocrinol Metab. 2019; 316:E1081–92.
Article19. Yu J, Zheng J, Liu XF, Feng ZL, Zhang XP, Cao LL, et al. Exercise improved lipid metabolism and insulin sensitivity in rats fed a high-fat diet by regulating glucose transporter 4 (GLUT4) and musclin expression. Braz J Med Biol Res. 2016; 49:e5129.
Article20. Deng Y, Tang Z. Research on intervention effects of musclin content in type 2 diabetic rats by aerobic exercise. Zhejiang Sport Sci. 2012; 34:113–5.21. Subbotina E, Sierra A, Zhu Z, Gao Z, Koganti SR, Reyes S, et al. Musclin is an activity-stimulated myokine that enhances physical endurance. Proc Natl Acad Sci U S A. 2015; 112:16042–7.
Article22. Re Cecconi AD, Forti M, Chiappa M, Zhu Z, Zingman LV, Cervo L, et al. Musclin, a myokine induced by aerobic exercise, retards muscle atrophy during cancer cachexia in mice. Cancers (Basel). 2019; 11:1541.
Article23. Jeremic N, Weber GJ, Theilen NT, Tyagi SC. Cardioprotective effects of high-intensity interval training are mediated through microRNA regulation of mitochondrial and oxidative stress pathways. J Cell Physiol. 2020; 235:5229–40.
Article24. Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009; 120:1640–5.25. Gallo Villegas JA, Ochoa Munera JE, Balparda Arias JK, Aristizabal Ocampo D. Cut points of waist circumference to identify subjects with insulin resistance in a colombian population. Acta Med Colomb. 2013; 38:118–6.26. Gallo-Villegas J, Aristizabal JC, Estrada M, Valbuena LH, Narvaez-Sanchez R, Osorio J, et al. Efficacy of high-intensity, low-volume interval training compared to continuous aerobic training on insulin resistance, skeletal muscle structure and function in adults with metabolic syndrome: study protocol for a randomized controlled clinical trial (Intraining-MET). Trials. 2018; 19:144.
Article27. Bull FC, Maslin TS, Armstrong T. Global physical activity questionnaire (GPAQ): nine country reliability and validity study. J Phys Act Health. 2009; 6:790–804.
Article28. Mancia G, De Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, et al. 2007 Guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2007; 25:1105–87.29. Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004; 27:1487–95.
Article30. Jelleyman C, Yates T, O’Donovan G, Gray LJ, King JA, Khunti K, et al. The effects of high-intensity interval training on glucose regulation and insulin resistance: a meta-analysis. Obes Rev. 2015; 16:942–61.
Article31. Mattioni Maturana F, Martus P, Zipfel S, Nieb AM. Effectiveness of HIIE versus MICT in improving cardiometabolic risk factors in health and disease: a meta-analysis. Med Sci Sports Exerc. 2021; 53:559–73.
Article32. Su L, Fu J, Sun S, Zhao G, Cheng W, Dou C, et al. Effects of HIIT and MICT on cardiovascular risk factors in adults with overweight and/or obesity: a meta-analysis. PLoS One. 2019; 14:e0210644.
Article33. Moreira OC, Alonso-Aubin DA, Patrocinio de Oliveira CE, Candia-Lujan R. Methods of assessment of body composition: an updated review of description, application, advantages and disadvantages. Arch Med Dep. 2015; 32:387–94.34. Sutter T, Duboeuf F, Chapurlat R, Cortet B, Lespessailles E, Roux JP. DXA body composition corrective factors between Hologic Discovery models to conduct multicenter studies. Bone. 2021; 142:115683.
Article35. Micklesfield LK, Goedecke JH, Punyanitya M, Wilson KE, Kelly TL. Dual-energy X-ray performs as well as clinical computed tomography for the measurement of visceral fat. Obesity (Silver Spring). 2012; 20:1109–14.
Article36. Neeland IJ, Grundy SM, Li X, Adams-Huet B, Vega GL. Comparison of visceral fat mass measurement by dual-X-ray absorptiometry and magnetic resonance imaging in a multiethnic cohort: the Dallas Heart Study. Nutr Diabetes. 2016; 6:e221.
Article37. Visser M, Fuerst T, Lang T, Salamone L, Harris TB. Validity of fan-beam dual-energy X-ray absorptiometry for measuring fat-free mass and leg muscle mass: Health, Aging, and Body Composition Study. Dual-Energy X-ray Absorptiometry and Body Composition Working Group. J Appl Physiol (1985). 1999; 87:1513–20.38. Baguet A, Everaert I, Hespel P, Petrovic M, Achten E, Derave W. A new method for non-invasive estimation of human muscle fiber type composition. PLoS One. 2011; 6:e21956.
Article39. Estrada M, Vega G, Reyngoudt H, Ricaurte G, Cardona OM, Gallo J, et al. Intramuscular absolute carnosine quantification in young athletes by 1H-MRS using a flexible coil. Skelet Radiol. 2016; 45:1021–9.40. Krssak M, Lindeboom L, Schrauwen-Hinderling V, Szczepaniak LS, Derave W, Lundbom J, et al. Proton magnetic resonance spectroscopy in skeletal muscle: experts’ consensus recommendations. NMR Biomed. 2021; 34:e4266.41. Olsen DB, Sacchetti M, Dela F, Ploug T, Saltin B. Glucose clearance is higher in arm than leg muscle in type 2 diabetes. J Physiol. 2005; 565(Pt 2):555–62.
Article42. Stegen S, Everaert I, Deldicque L, Vallova S, de Courten B, Ukropcova B, et al. Muscle histidine-containing dipeptides are elevated by glucose intolerance in both rodents and men. PLoS One. 2015; 10:e0121062.
Article43. Vanhamme L, van den Boogaart A, Van Huffel S. Improved method for accurate and efficient quantification of MRS data with use of prior knowledge. J Magn Reson. 1997; 129:35–43.
Article44. Just Kukurova I, Valkovic L, Ukropec J, de Courten B, Chmelik M, Ukropcova B, et al. Improved spectral resolution and high reliability of in vivo (1) H MRS at 7 T allow the characterization of the effect of acute exercise on carnosine in skeletal muscle. NMR Biomed. 2016; 29:24–32.45. Luong Q, Huang J, Lee KY. Deciphering white adipose tissue heterogeneity. Biology (Basel). 2019; 8:23.
Article46. Chait A, den Hartigh LJ. Adipose tissue distribution, inflammation and its metabolic consequences, including diabetes and cardiovascular disease. Front Cardiovasc Med. 2020; 7:22.
Article47. Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu CY, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007; 116:39–48.
Article48. Wu H, Qi Q, Yu Z, Sun Q, Wang J, Franco OH, et al. Independent and opposite associations of trunk and leg fat depots with adipokines, inflammatory markers, and metabolic syndrome in middle-aged and older Chinese men and women. J Clin Endocrinol Metab. 2010; 95:4389–98.
Article49. Zhang M, Hu T, Zhang S, Zhou L. Associations of different adipose tissue depots with insulin resistance: a systematic review and meta-analysis of observational studies. Sci Rep. 2015; 5:18495.
Article50. Gabriely I, Ma XH, Yang XM, Atzmon G, Rajala MW, Berg AH, et al. Removal of visceral fat prevents insulin resistance and glucose intolerance of aging: an adipokine-mediated process? Diabetes. 2002; 51:2951–8.51. Tran TT, Yamamoto Y, Gesta S, Kahn CR. Beneficial effects of subcutaneous fat transplantation on metabolism. Cell Metab. 2008; 7:410–20.
Article52. Jensen MD, Haymond MW, Rizza RA, Cryer PE, Miles JM. Influence of body fat distribution on free fatty acid metabolism in obesity. J Clin Invest. 1989; 83:1168–73.
Article53. Chavez JA, Summers SA. Characterizing the effects of saturated fatty acids on insulin signaling and ceramide and diacylglycerol accumulation in 3T3-L1 adipocytes and C2C12 myotubes. Arch Biochem Biophys. 2003; 419:101–9.
Article54. Bermudez-Cardona J, Velasquez-Rodriguez C. Profile of free fatty acids and fractions of phospholipids, cholesterol esters and triglycerides in serum of obese youth with and without metabolic syndrome. Nutrients. 2016; 8:54.
Article55. Boden G. Obesity and free fatty acids. Endocrinol Metab Clin North Am. 2008; 37:635–46.
Article56. Thomas G, Moffatt P, Salois P, Gaumond MH, Gingras R, Godin E, et al. Osteocrin, a novel bone-specific secreted protein that modulates the osteoblast phenotype. J Biol Chem. 2003; 278:50563–71.
Article57. Bouchard C, Hoffman E. Genetic and molecular aspects of sport performance. 18th ed. Chichester: Blackwell Publishing;2011. Chapter 16:Genetic determinants of exercise performance: evidence from transgenic and null mouse models. p. 185–94.58. He L. Metformin and systemic metabolism. Trends Pharmacol Sci. 2020; 41:868–81.
Article59. Verdijk LB, Koopman R, Schaart G, Meijer K, Savelberg HH, van Loon LJ. Satellite cell content is specifically reduced in type II skeletal muscle fibers in the elderly. Am J Physiol Endocrinol Metab. 2007; 292:E151–7.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Association of Cardiorespiratory Fitness with Insulin Resistance, Blood Lipids and Bone Mineral Density in Young Female Adults
- Association between Estimated Cardiorespiratory Fitness and Insulin Resistance in Korean Adults: Results from the Korea National Health and Nutrition Survey 2019–2021
- Importance of Lean Muscle Maintenance to Improve Insulin Resistance by Body Weight Reduction in Female Patients with Obesity
- Body Composition Analysis in Newly Diagnosed Diabetic Adolescent Girls
- Fibroblast PC-1 mRNA Content, Body mass index and Insulin Sensitivity in Korean NIDDM Patients