Endocrinol Metab.
2021 Oct;36(5):988-996. 10.3803/EnM.2021.1107.
How Can We Adopt the Glucose Tolerance Test to Facilitate Predicting Pregnancy Outcome in Gestational Diabetes Mellitus?
- Affiliations
-
- 1Division of Endocrinology and Metabolism, Department of Internal Medicine, Korea University College of Medicine, Seoul, Korea
- 2Department of Obstetrics and Gynecology, Korea University College of Medicine, Seoul, Korea
- KMID: 2521947
- DOI: http://doi.org/10.3803/EnM.2021.1107
Abstract
- Background
We investigated how 100-g oral glucose tolerance test (OGTT) results can be used to predict adverse pregnancy outcomes in gestational diabetes mellitus (GDM) patients.
Methods
We analyzed 1,059 pregnant women who completed the 100-g OGTT between 24 and 28 weeks of gestation. We compared the risk of adverse pregnancy outcomes according to OGTT patterns by latent profile analysis (LPA), numbers to meet the OGTT criteria, and area under the curve (AUC) of the OGTT graph. Adverse pregnancy outcomes were defined as a composite of preterm birth, macrosomia, large for gestational age, low APGAR score at 1 minute, and pregnancy-induced hypertension.
Results
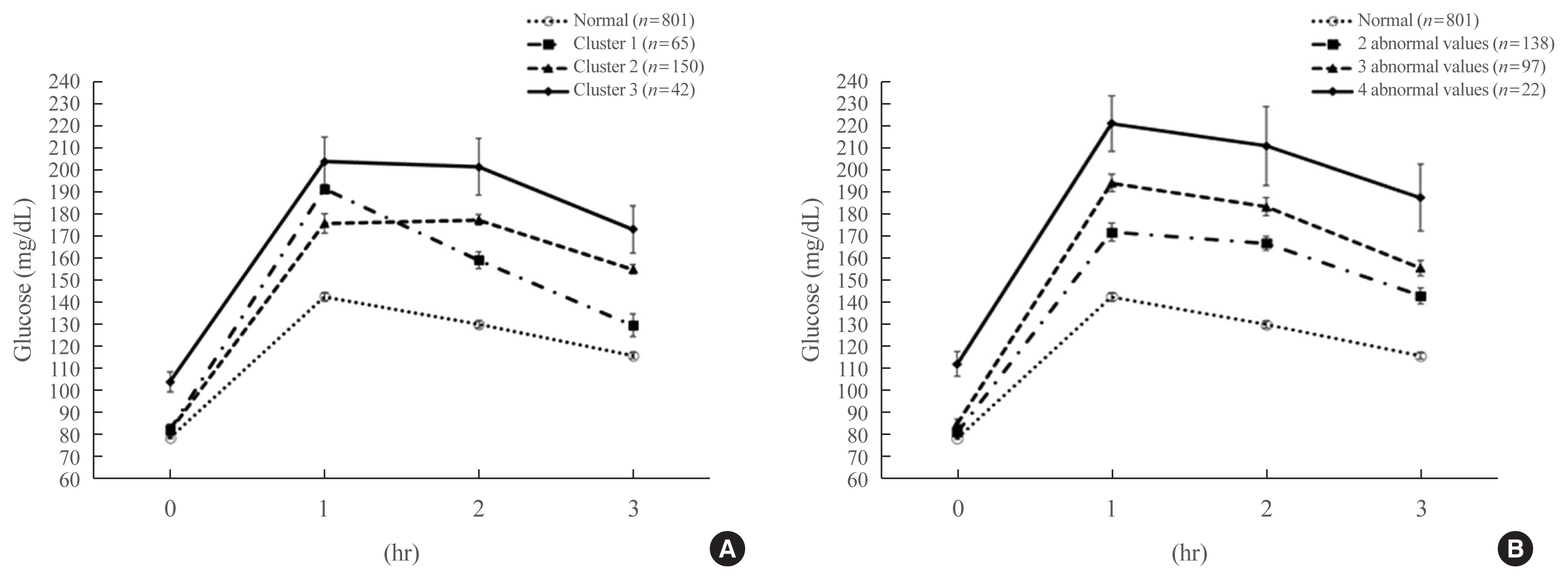
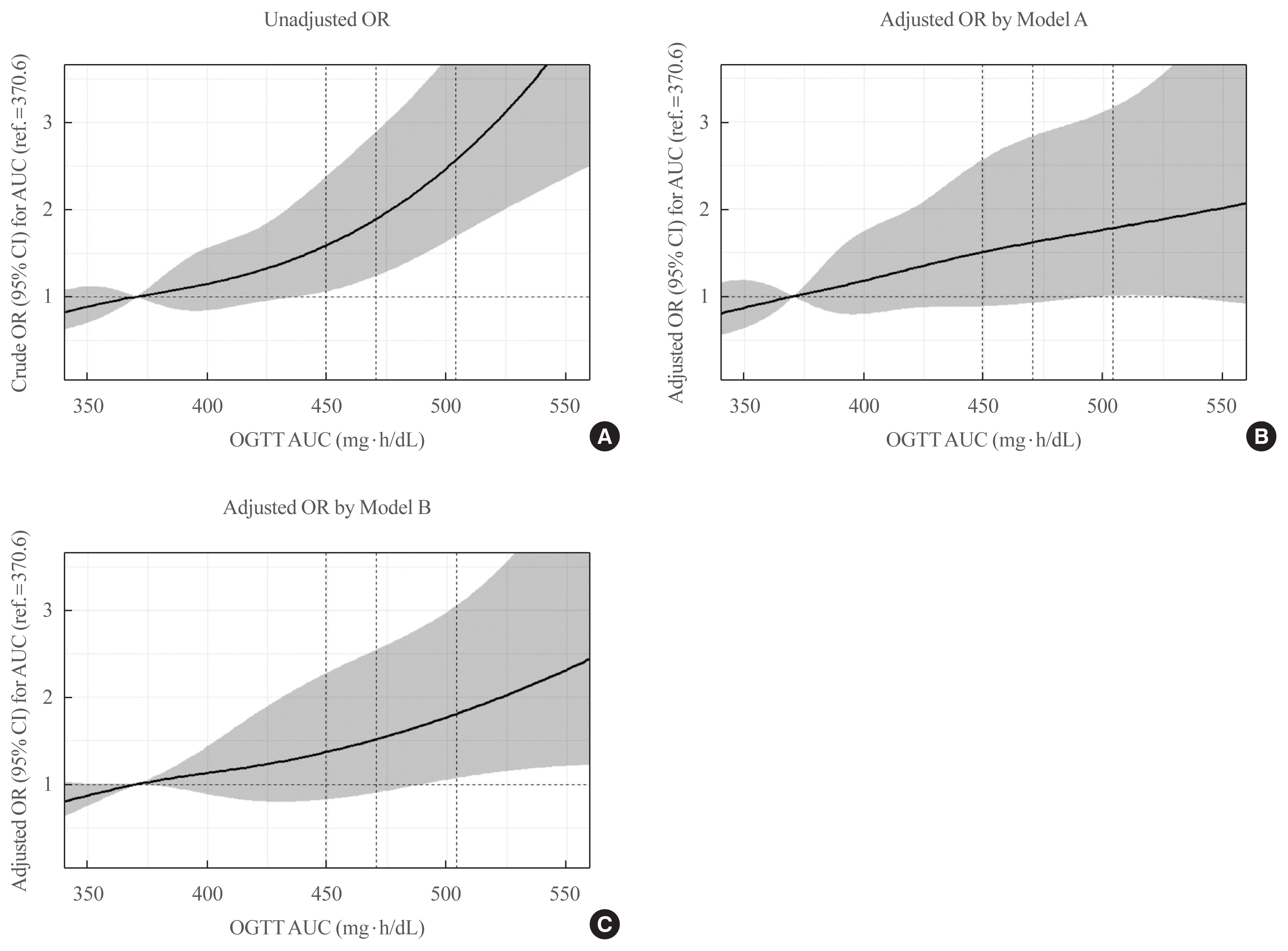
Overall, 257 participants were diagnosed with GDM, with a median age of 34 years. An LPA led to three different clusters of OGTT patterns; however, there were no significant associations between the clusters and adverse pregnancy outcomes after adjusting for confounders. Notwithstanding, the risk of adverse pregnancy outcome increased with an increase in number to meet the OGTT criteria (P for trend=0.011); odds ratios in a full adjustment model were 1.27 (95% confidence interval [CI], 0.72 to 2.23), 2.16 (95% CI, 1.21 to 3.85), and 2.32 (95% CI, 0.66 to 8.15) in those meeting the 2, 3, and 4 criteria, respectively. The AUCs of the OGTT curves also distinguished the patients at risk of adverse pregnancy outcomes; the larger the AUC, the higher the risk (P for trend=0.007).
Conclusion
The total number of abnormal values and calculated AUCs for the 100-g OGTT may facilitate tailored management of patients with GDM by predicting adverse pregnancy outcomes.
Figure
Reference
-
1. American Diabetes Association. 6. Glycemic targets: standards of medical care in diabetes-2020. Diabetes Care. 2020; 43(Suppl 1):S66–76.2. Magliano DJ, Islam RM, Barr ELM, Gregg EW, Pavkov ME, Harding JL, et al. Trends in incidence of total or type 2 diabetes: systematic review. BMJ. 2019; 366:l5003.
Article3. World Health Organization. Diabetes [Internet]. Geneva: WHO;2021. [cited 2021 Sep 16]. Available from: https://www.who.int/news-room/fact-sheets/detail/diabetes .4. Kim BY, Won JC, Lee JH, Kim HS, Park JH, Ha KH, et al. Diabetes fact sheets in Korea, 2018: an appraisal of current status. Diabetes Metab J. 2019; 43:487–94.
Article5. Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol. 1982; 144:768–73.
Article6. HAPO Study Cooperative Research Group. Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008; 358:1991–2002.
Article7. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. National Diabetes Data Group. Diabetes. 1979; 28:1039–57.8. International Association of Diabetes and Pregnancy Study Groups Consensus Panel. Metzger BE, Gabbe SG, Persson B, Buchanan TA, Catalano PA, et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010; 33:676–82.
Article9. Vandorsten JP, Dodson WC, Espeland MA, Grobman WA, Guise JM, Mercer BM, et al. NIH consensus development conference: diagnosing gestational diabetes mellitus. NIH Consens State Sci Statements. 2013; 29:1–31.10. Caughey AB. Gestational diabetes mellitus. Obstet Gynecol. 2017; 130:E17–31.11. Kim MK, Ko SH, Kim BY, Kang ES, Noh J, Kim SK, et al. 2019 Clinical practice guidelines for type 2 diabetes mellitus in Korea. Diabetes Metab J. 2019; 43:398–406.
Article12. McIntyre HD, Catalano P, Zhang C, Desoye G, Mathiesen ER, Damm P. Gestational diabetes mellitus. Nat Rev Dis Primers. 2019; 5:47.
Article13. Buchanan TA, Xiang AH, Page KA. Gestational diabetes mellitus: risks and management during and after pregnancy. Nat Rev Endocrinol. 2012; 8:639–49.
Article14. Gruendhammer M, Brezinka C, Lechleitner M. The number of abnormal plasma glucose values in the oral glucose tolerance test and the feto-maternal outcome of pregnancy. Eur J Obstet Gynecol Reprod Biol. 2003; 108:131–6.
Article15. Kim HS, Chang KH, Yang JI, Yang SC, Lee HJ, Ryu HS. Clinical outcomes of pregnancy with one elevated glucose tolerance test value. Int J Gynaecol Obstet. 2002; 78:131–8.
Article16. Korean Diabetes Association. 2021 Clinical practice guideline for diabetes. 7th ed. Seoul: KDA;2021. Available from: https://www.diabetes.or.kr/pro/publish/guide.php?code=guide&number=853&mode=view .17. Hur J, Cho EH, Baek KH, Lee KJ. Prediction of gestational diabetes mellitus by unconjugated estriol levels in maternal serum. Int J Med Sci. 2017; 14:123–7.
Article18. Cho EH, Hur J, Lee KJ. Early gestational weight gain rate and adverse pregnancy outcomes in Korean women. PLoS One. 2015; 10:e0140376.
Article19. Van De Schoot R, Sijbrandij M, Winter SD, Depaoli S, Vermunt JK. The GRoLTS-checklist: guidelines for reporting on latent trajectory studies. Struct Equ Modeling. 2017; 24:451–67.
Article20. Hulman A, Vistisen D, Glumer C, Bergman M, Witte DR, Faerch K. Glucose patterns during an oral glucose tolerance test and associations with future diabetes, cardiovascular disease and all-cause mortality rate. Diabetologia. 2018; 61:101–7.
Article21. Scrucca L, Fop M, Murphy TB, Raftery AE. Mclust 5: clustering, classification and density estimation using gaussian finite mixture models. R J. 2016; 8:289–317.
Article22. International Diabetes Federation. IDF diabetes atlas. 9th ed.Brussels: IDF;2020. Available from: https://diabetesatlas.org/en .23. Kim S, Min WK, Chun S, Lee W, Chung HJ, Lee PR, et al. Quantitative risk estimation for large for gestational age using the area under the 100-g oral glucose tolerance test curve. J Clin Lab Anal. 2009; 23:231–6.
Article24. Zhang C, Wei Y, Sun W, Yang H. The area under the curve (AUC) of oral glucose tolerance test (OGTT) could be a measure method of hyperglycemia in all pregnant women. Open J Obstet Gynecol. 2019; 9:186–95.
Article25. Kim AM, Park JH, Kang S, Yoon TH, Kim Y. An ecological study of geographic variation and factors associated with cesarean section rates in South Korea. BMC Pregnancy Childbirth. 2019; 19:162.
Article26. Betran AP, Ye J, Moller AB, Zhang J, Gulmezoglu AM, Torloni MR. The increasing trend in caesarean section rates: global, regional and national estimates: 1990–2014. PLoS One. 2016; 11:e0148343.
Article27. Chung SH, Seol HJ, Choi YS, Oh SY, Kim A, Bae CW. Changes in the cesarean section rate in Korea (1982–2012) and a review of the associated factors. J Korean Med Sci. 2014; 29:1341–52.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Gestational Diabetes Mellitus: Diagnostic Approaches and Maternal-Offspring Complications
- Update in management of gestational diabetes mellitus
- Glucose Metabolism in Pregnant Women with Normal Glucose Tolerance
- Risk Factors of Gestational Diabetes Mellitus and their Significances
- Gestational Diabetes Mellitus: Diagnosis and Glycemic Control