Intest Res.
2021 Oct;19(4):360-364. 10.5217/ir.2020.00116.
Gut microbiome and checkpoint inhibitor colitis
- Affiliations
-
- 1Division of Gastroenterology and Hepatology, Mayo Clinic, Rochester, MN, USA
- KMID: 2521599
- DOI: http://doi.org/10.5217/ir.2020.00116
Abstract
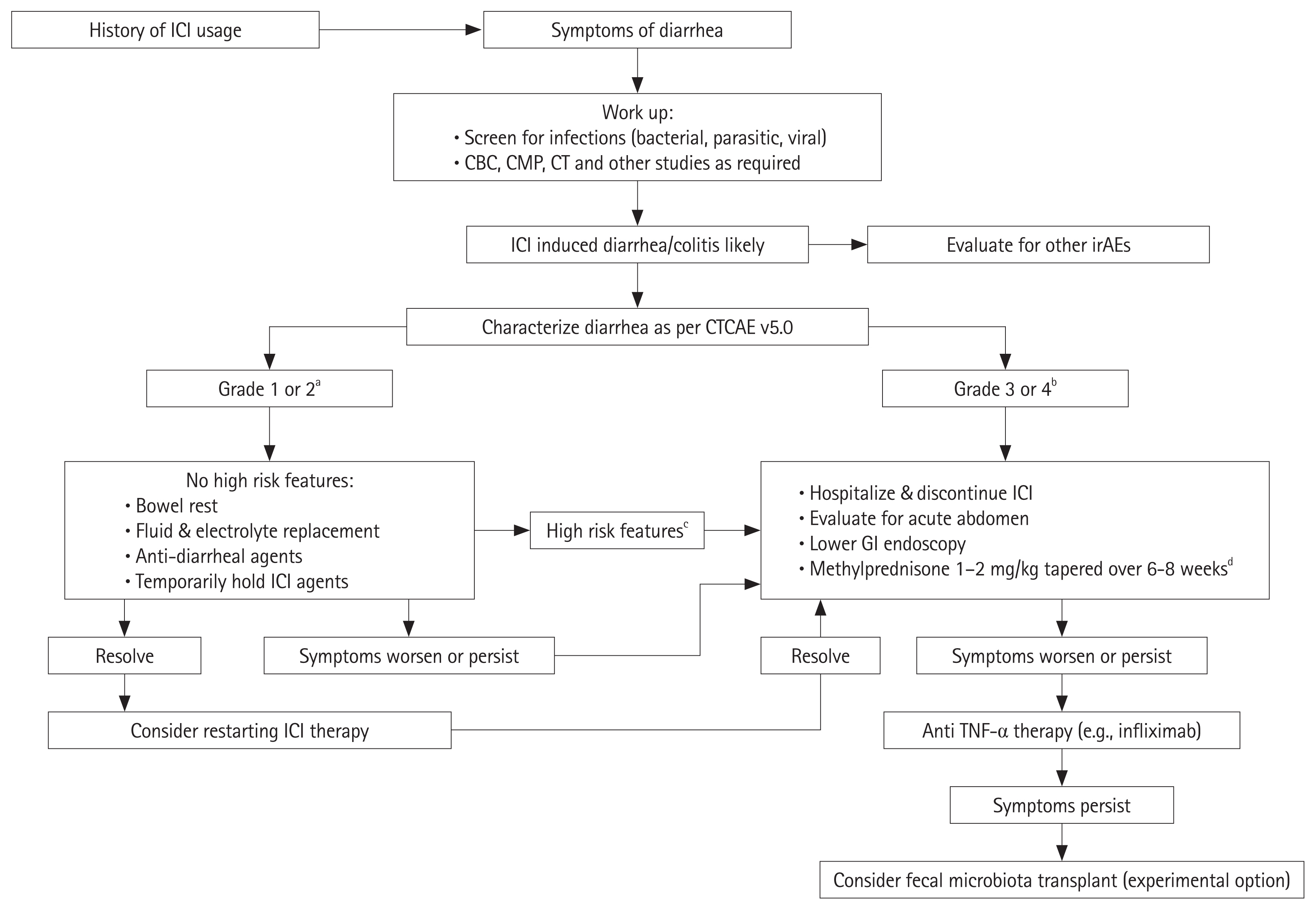
- Immune checkpoint inhibitor therapies such as ipilimumab, are increasingly being used as a treatment option for a variety of cancers, including metastatic melanoma and have demonstrated effectively a prolonged survival. These agents have an immunological mode of action that predisposes patients to a number of immune-related adverse events, colitis being one of the most commonly encountered complications. The pathogenesis for the development of colitis is unclear, and there is a growing consensus that the ecosystem of the gastrointestinal microbiota plays a significant role. Based on this suspected connection, studies are being carried out to explore the changes in the microbiota in patients on these medications who develop colitis. Conceivably, the modulation of the gut microbiota could offer a therapeutic benefit. Fecal microbiota transplantation is one therapeutic option that is currently being investigated, though there are still more data needed to evaluate its efficacy. In this review, we recapitulate the mechanisms of action of immune checkpoint inhibitors, their adverse events, with a focus on colitis and the role gut microbiota are suspected to play, and finally discuss the microbiota modulation therapies being investigated.
Keyword
Figure
Cited by 2 articles
-
Compositional changes in fecal microbiota associated with clinical phenotypes and prognosis in Korean patients with inflammatory bowel disease
Seung Yong Shin, Young Kim, Won-Seok Kim, Jung Min Moon, Kang-Moon Lee, Sung-Ae Jung, Hyesook Park, Eun Young Huh, Byung Chang Kim, Soo Chan Lee, Chang Hwan Choi
Intest Res. 2023;21(1):148-160. doi: 10.5217/ir.2021.00168.Gut Microbiome and Colorectal Cancer
Tae-Geun Gweon
Korean J Gastroenterol. 2023;82(2):56-62. doi: 10.4166/kjg.2023.089.
Reference
-
1. Reddy HG, Schneider BJ, Tai AW. Immune checkpoint inhibitor-associated colitis and hepatitis. Clin Transl Gastroenterol. 2018; 9:180.
Article2. Abu-Sbeih H, Wang Y. Gut microbiome and immune checkpoint inhibitor-induced enterocolitis. Dig Dis Sci. 2020; 65:797–799.
Article3. Gupta A, De Felice KM, Loftus EV Jr, Khanna S. Systematic review: colitis associated with anti-CTLA-4 therapy. Aliment Pharmacol Ther. 2015; 42:406–417.
Article4. Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med. 2018; 378:158–168.
Article5. Lacroix M. Targeted therapies in cancer. Berlin: Springer;2014.6. Singh BP, Marshall JL, He AR. Workup and management of immune-mediated colitis in patients treated with immune checkpoint inhibitors. Oncologist. 2020; 25:197–202.
Article7. Wang DY, Salem JE, Cohen JV, et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. 2018; 4:1721–1728.
Article8. Kumar V, Chaudhary N, Garg M, Floudas CS, Soni P, Chandra AB. current diagnosis and management of immune related adverse events (irAEs) induced by immune checkpoint inhibitor therapy. Front Pharmacol. 2017; 8:49.9. Michot JM, Bigenwald C, Champiat S, et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer. 2016; 54:139–148.10. Som A, Mandaliya R, Alsaadi D, et al. Immune checkpoint inhibitor-induced colitis: a comprehensive review. World J Clin Cases. 2019; 7:405–418.
Article11. Read S, Malmström V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J Exp Med. 2000; 192:295–302.12. Chaput N, Lepage P, Coutzac C, et al. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann Oncol. 2019; 30:2012.
Article13. Dubin K, Callahan MK, Ren B, et al. Intestinal microbiome analyses identify melanoma patients at risk for checkpoint-blockade-induced colitis. Nat Commun. 2016; 7:10391.14. Dutta D, Lim SH. Bidirectional interaction between intestinal microbiome and cancer: opportunities for therapeutic interventions. Biomark Res. 2020; 8:31.
Article15. Salgia NJ, Bergerot PG, Maia MC, et al. Stool microbiome profiling of patients with metastatic renal cell carcinoma receiving anti-PD-1 immune checkpoint inhibitors. Eur Urol. 2020; 78:498–502.
Article16. Routy B, Gopalakrishnan V, Daillère R, Zitvogel L, Wargo JA, Kroemer G. The gut microbiota influences anticancer immunosurveillance and general health. Nat Rev Clin Oncol. 2018; 15:382–396.17. Friedman CF, Proverbs-Singh TA, Postow MA. Treatment of the immune-related adverse effects of immune checkpoint inhibitors: a review. JAMA Oncol. 2016; 2:1346–1353.
Article18. Abu-Sbeih H, Ali FS, Wang Y. Immune-checkpoint inhibitors induced diarrhea and colitis: a review of incidence, pathogenesis and management. Curr Opin Gastroenterol. 2020; 36:25–32.19. Marthey L, Mateus C, Mussini C, et al. Cancer immunotherapy with anti-CTLA-4 monoclonal antibodies induces an inflammatory bowel disease. J Crohns Colitis. 2016; 10:395–401.20. Iranzo I, Huguet JM, Suárez P, Ferrer-Barceló L, Iranzo V, Sempere J. Endoscopic evaluation of immunotherapy-induced gastrointestinal toxicity. World J Gastrointest Endosc. 2018; 10:392–399.21. Verschuren EC, van den Eertwegh AJ, Wonders J, et al. Clinical, endoscopic, and histologic characteristics of ipilimumab-associated colitis. Clin Gastroenterol Hepatol. 2016; 14:836–842.
Article22. Common terminology criteria for adverse events (CTCAE) version 5.0. National Cancer Institute Web site. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htmUpdated November 27, 2017. Accessed November 10, 2020.23. Wang Y, Wiesnoski DH, Helmink BA, et al. Fecal microbiota transplantation for refractory immune checkpoint inhibitor-associated colitis. Nat Med. 2018; 24:1804–1808.
Article24. Wang F, Yin Q, Chen L, Davis MM. Bifidobacterium can mitigate intestinal immunopathology in the context of CTLA-4 blockade. Proc Natl Acad Sci U S A. 2018; 115:157–161.
Article25. Fasanello MK, Robillard KT, Boland PM, Bain AJ, Kanehira K. Use of fecal microbial transplantation for immune checkpoint inhibitor colitis. ACG Case Rep J. 2020; 7:e00360.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Gut microbiome on immune checkpoint inhibitor therapy and consequent immune-related colitis: a review
- Complex influences of gut microbiome metabolism on various drug responses
- Mental Disorders Linked to Crosstalk between The Gut Microbiome and The Brain
- Early Life Events and Development of Gut Microbiota in Infancy
- Microbiome and Diabetes