Cancer Res Treat.
2021 Oct;53(4):991-1003. 10.4143/crt.2020.1298.
Prognostic Value of Serum Epstein-Barr Virus Antibodies and Their Correlation with TNM Classification in Patients with Locoregionally Advanced Nasopharyngeal Carcinoma
- Affiliations
-
- 1Department of Nasopharyngeal Carcinoma, Sun Yat‐sen University Cancer Center, Guangzhou, China
- KMID: 2521575
- DOI: http://doi.org/10.4143/crt.2020.1298
Abstract
- Purpose
This study assessed the correlation between Epstein-Barr virus (EBV) biomarkers and the eighth American Joint Committee on Cancer staging system and the prognostic values of IgG antibodies against replication and transcription activator (Rta-IgG), IgA antibodies against Epstein-Barr nuclear antigen 1, and BamH1 Z transactivator (Zta-IgA) in locoregionally advanced nasopharyngeal carcinoma (NPC) patients.
Materials and Methods
Serum EBV antibody levels were measured by enzyme-linked immunosorbent assay in 435 newly diagnosed stage III-IVA NPC patients administered intensity-modulated radiation therapy±chemotherapy. The primary endpoint was progression-free survival (PFS).
Results
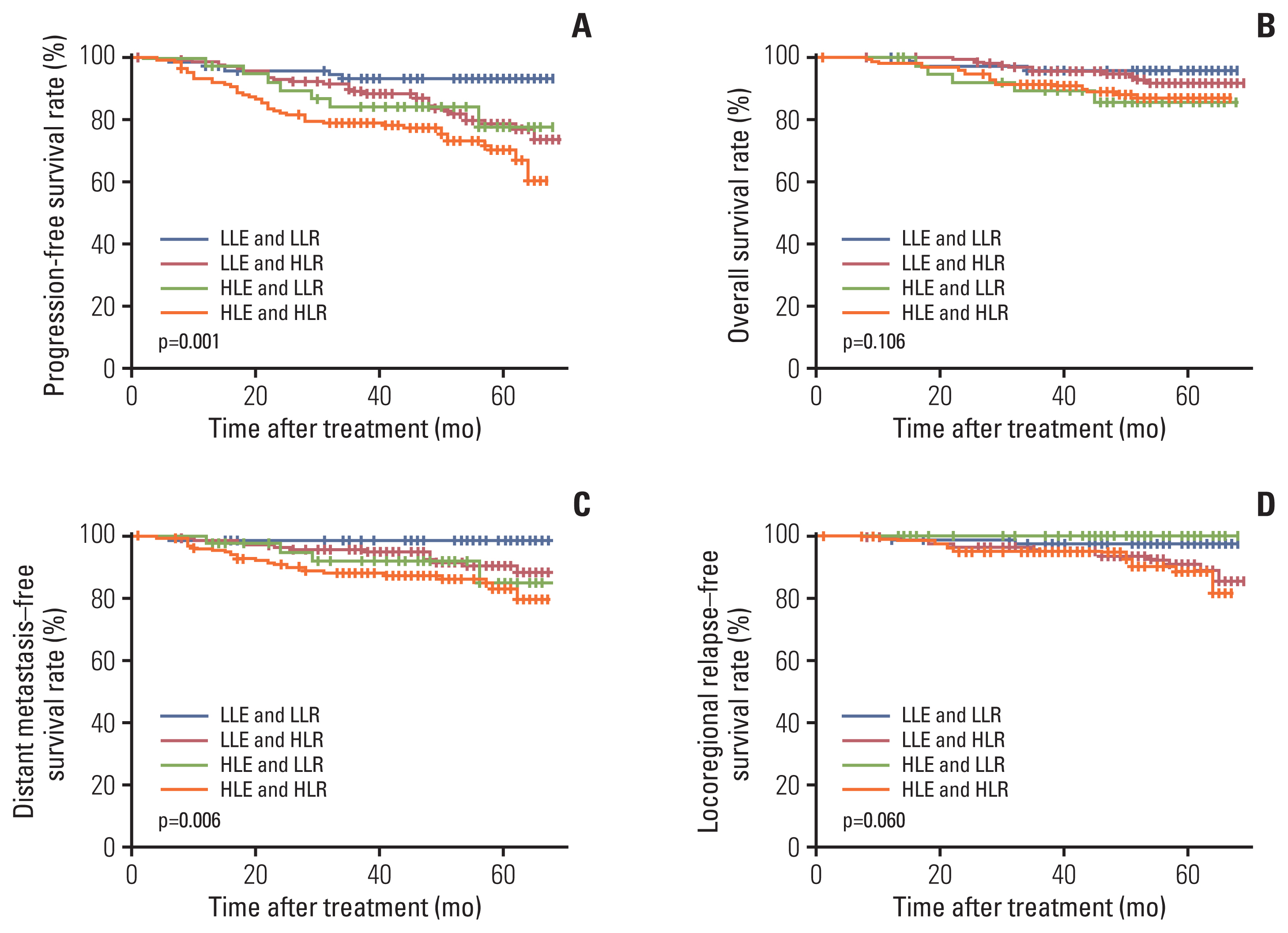
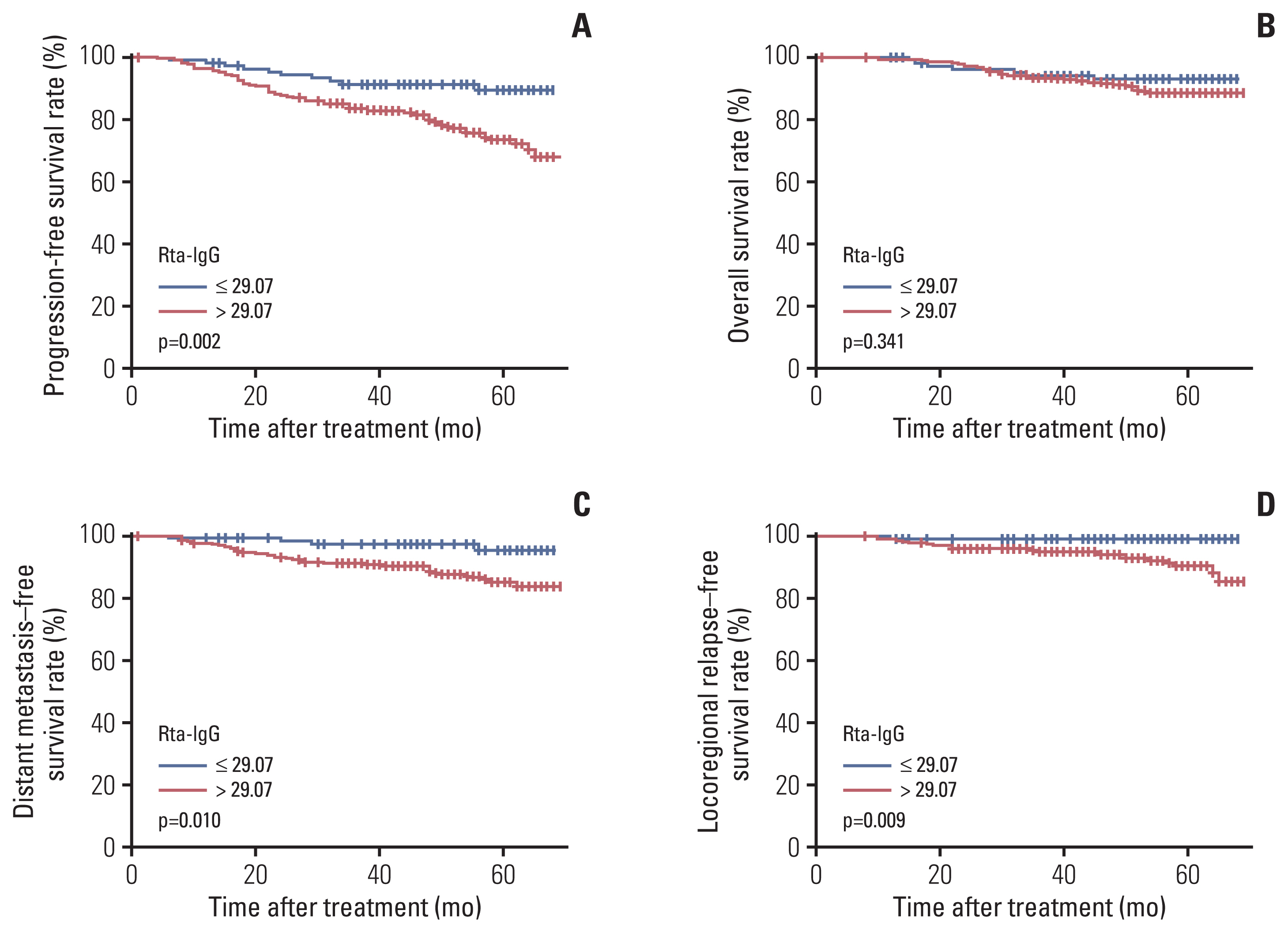
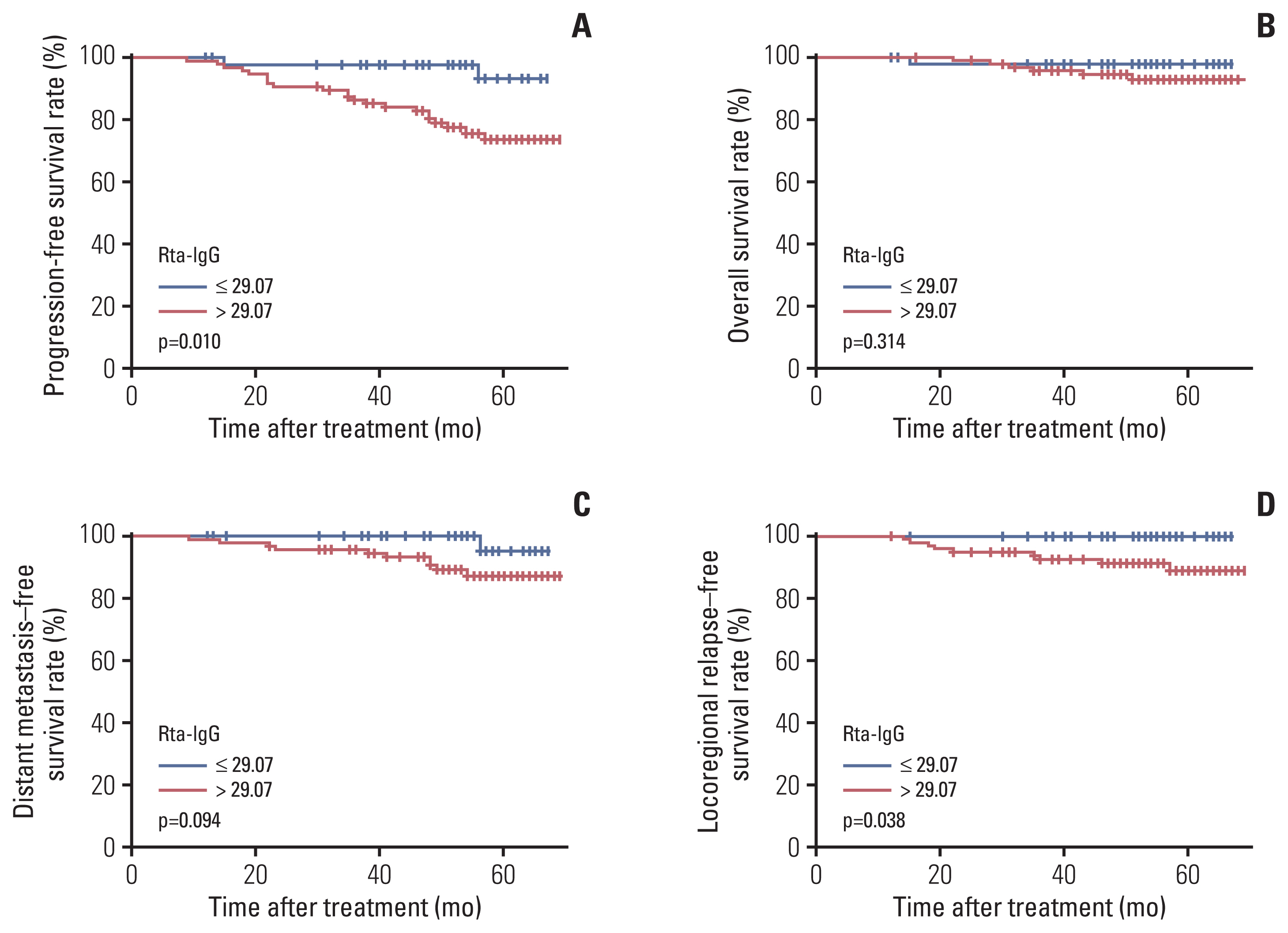
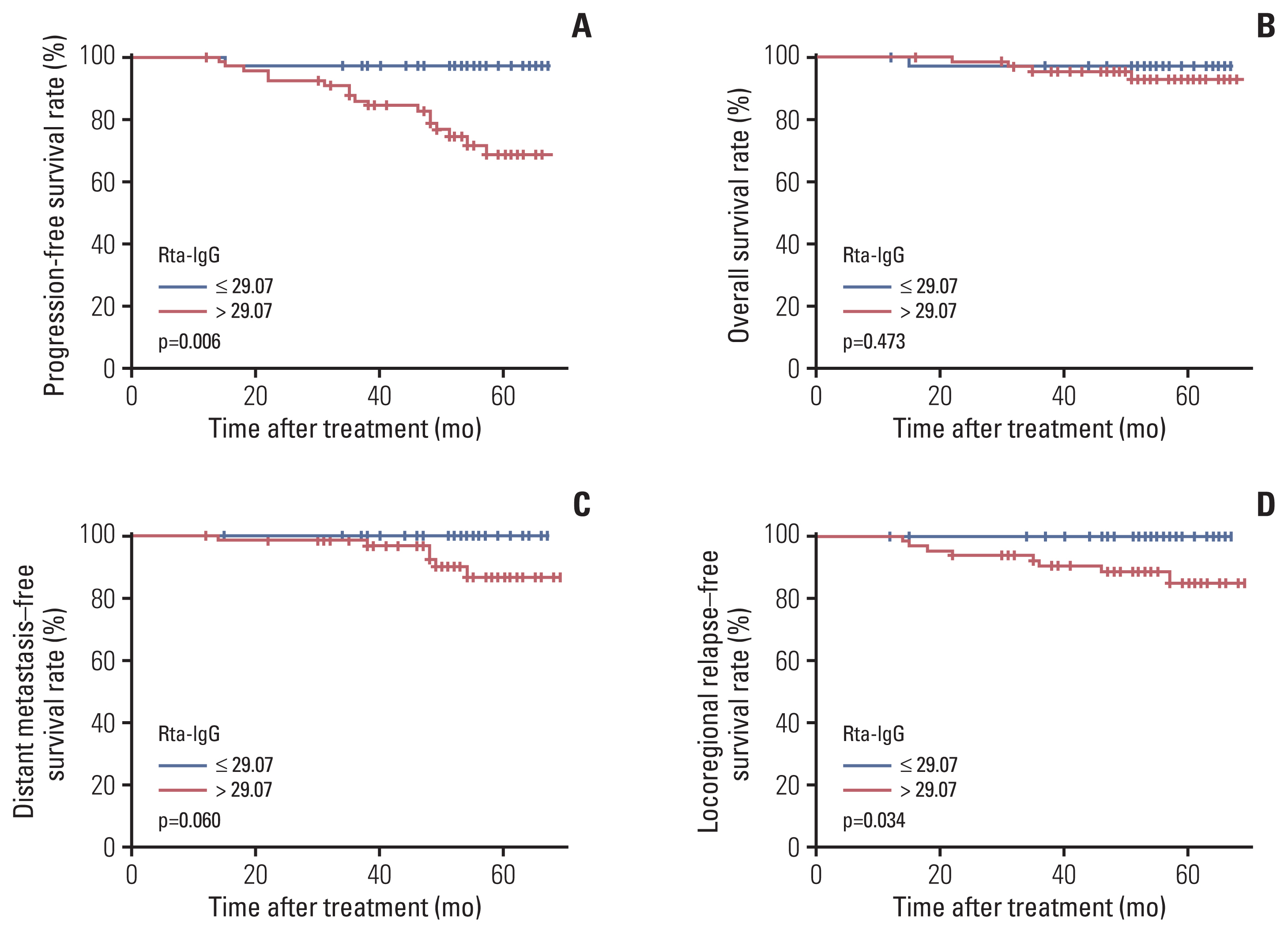
Rta-IgG and Zta-IgA levels were positively correlated with the N category and clinical stage. Patients with high Rta-IgG levels (> 29.07 U/mL) showed a significantly inferior prognosis as indicated by PFS (77% vs. 89.8%, p=0.004), distant metastasis–free survival (DMFS) (88.3% vs. 95.8%, p=0.021), and local recurrence-free survival (LRFS) (91.2% vs. 98.3%, p=0.009). High Rta-IgG levels were also significantly associated with inferior PFS and LRFS in multivariable analyses. In the low-level EBV DNA group (≤ 1,500 copies/mL), patients with high Rta-IgG levels had significantly inferior PFS and DMFS (both p < 0.05). However, in the high-level EBV DNA group, Rta-IgG levels were not significantly associated with PFS, DMFS, and LRFS. In the advanced T category (T3-4) subgroup, high Rta-IgG levels were also significantly associated with inferior PFS, DMFS, and LRFS (both p < 0.05).
Conclusion
Rta-IgG and Zta-IgA levels were strongly correlated with the TNM classification. Rta-IgG level was a negative prognostic factor in locoregionally advanced NPC patients, especially those with advanced T category or low EBV DNA level.
Figure
Reference
-
References
1. Yu WM, Hussain SS. Incidence of nasopharyngeal carcinoma in Chinese immigrants, compared with Chinese in China and South East Asia: review. J Laryngol Otol. 2009; 123:1067–74.
Article2. Chang ET, Adami HO. The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev. 2006; 15:1765–77.
Article3. Yi JL, Gao L, Huang XD, Li SY, Luo JW, Cai WM, et al. Nasopharyngeal carcinoma treated by radical radiotherapy alone: Ten-year experience of a single institution. Int J Radiat Oncol Biol Phys. 2006; 65:161–8.
Article4. Leung SF, Chan AT, Zee B, Ma B, Chan LY, Johnson PJ, et al. Pretherapy quantitative measurement of circulating Epstein-Barr virus DNA is predictive of posttherapy distant failure in patients with early-stage nasopharyngeal carcinoma of undifferentiated type. Cancer. 2003; 98:288–91.
Article5. Leung SF, Zee B, Ma BB, Hui EP, Mo F, Lai M, et al. Plasma Epstein-Barr viral deoxyribonucleic acid quantitation complements tumor-node-metastasis staging prognostication in nasopharyngeal carcinoma. J Clin Oncol. 2006; 24:5414–8.
Article6. Gu AD, Zeng MS, Qian CN. The criteria to confirm the role of Epstein-Barr virus in nasopharyngeal carcinoma initiation. Int J Mol Sci. 2012; 13:13737–47.
Article7. Leung SF, Chan KC, Ma BB, Hui EP, Mo F, Chow KC, et al. Plasma Epstein-Barr viral DNA load at midpoint of radiotherapy course predicts outcome in advanced-stage nasopharyngeal carcinoma. Ann Oncol. 2014; 25:1204–8.
Article8. Sun P, Chen C, Cheng YK, Zeng ZJ, Chen XL, Liu LZ, et al. Serologic biomarkers of Epstein-Barr virus correlate with TNM classification according to the seventh edition of the UICC/AJCC staging system for nasopharyngeal carcinoma. Eur Arch Otorhinolaryngol. 2014; 271:2545–54.
Article9. Shao JY, Li YH, Gao HY, Wu QL, Cui NJ, Zhang L, et al. Comparison of plasma Epstein-Barr virus (EBV) DNA levels and serum EBV immunoglobulin A/virus capsid antigen antibody titers in patients with nasopharyngeal carcinoma. Cancer. 2004; 100:1162–70.10. Cai YL, Zheng YM, Cheng JR, Wang W, Zhang YN, Wang WH, et al. Relationship between clinical stages of nasopharyngeal carcinoma and Epstein-Barr virus antibodies Rta/IgG, EBNA1/IgA, VCA/IgA and EA/IgA. Nan Fang Yi Ke Da Xue Xue Bao. 2010; 30:509–11.11. Yao JJ, Lin L, Jin YN, Wang SY, Zhang WJ, Zhang F, et al. Prognostic value of serum Epstein-Barr virus antibodies in patients with nasopharyngeal carcinoma and undetectable pretreatment Epstein-Barr virus DNA. Cancer Sci. 2017; 108:1640–7.12. Lee AW, Lau KY, Hung WM, Ng WT, Lee MC, Choi CW, et al. Potential improvement of tumor control probability by induction chemotherapy for advanced nasopharyngeal carcinoma. Radiother Oncol. 2008; 87:204–10.
Article13. Lin S, Lu JJ, Han L, Chan Q, Pan J. Sequential chemotherapy and intensity-modulated radiation therapy in the management of locoregionally advanced nasopharyngeal carcinoma: experience of 370 consecutive cases. BMC Cancer. 2010; 10:39.
Article14. Xu FH, Xiong D, Xu YF, Cao SM, Xue WQ, Qin HD, et al. An epidemiological and molecular study of the relationship between smoking, risk of nasopharyngeal carcinoma, and Epstein-Barr virus activation. J Natl Cancer Inst. 2012; 104:1396–410.
Article15. Liu Y, Huang Q, Liu W, Liu Q, Jia W, Chang E, et al. Establishment of VCA and EBNA1 IgA-based combination by enzyme-linked immunosorbent assay as preferred screening method for nasopharyngeal carcinoma: a two-stage design with a preliminary performance study and a mass screening in southern China. Int J Cancer. 2012; 131:406–16.
Article16. Zeng Y, Zhang LG, Wu YC, Huang YS, Huang NQ, Li JY, et al. Prospective studies on nasopharyngeal carcinoma in Epstein-Barr virus IgA/VCA antibody-positive persons in Wuzhou City, China. Int J Cancer. 1985; 36:545–7.17. Ji MF, Wang DK, Yu YL, Guo YQ, Liang JS, Cheng WM, et al. Sustained elevation of Epstein-Barr virus antibody levels preceding clinical onset of nasopharyngeal carcinoma. Br J Cancer. 2007; 96:623–30.
Article18. Ling W, Cao SM, Huang QH, Li YH, Deng MQ. Prognostic implication of pretreatment titer of serum immunoglobulin A against Epstein-Barr virus capsid antigen in nasopharyngeal carcinoma patients in Sihui, Guangdong. Ai Zheng. 2009; 28:57–9.19. Sun R, Qiu HZ, Mai HQ, Zhang Q, Hong MH, Li YX, et al. Prognostic value and differences of the sixth and seventh editions of the UICC/AJCC staging systems in nasopharyngeal carcinoma. J Cancer Res Clin Oncol. 2013; 139:307–14.
Article20. Lo YM, Leung SF, Chan LY, Chan AT, Lo KW, Johnson PJ, et al. Kinetics of plasma Epstein-Barr virus DNA during radiation therapy for nasopharyngeal carcinoma. Cancer Res. 2000; 60:2351–5.21. Chen WH, Tang LQ, Guo SS, Chen QY, Zhang L, Liu LT, et al. Prognostic value of plasma Epstein-Barr virus DNA for local and regionally advanced nasopharyngeal carcinoma treated with cisplatin-based concurrent chemoradiotherapy in intensity-modulated radiotherapy era. Medicine (Baltimore). 2016; 95:e2642.
Article22. Du YY, Luo DH, Sun XS, Tang LQ, Mai HQ, Chen QY, et al. Combining pretreatment plasma Epstein-Barr virus DNA level and cervical node necrosis improves prognostic stratification in patients with nasopharyngeal carcinoma: a cohort study. Cancer Med. 2019; 8:6841–52.
Article23. Hau PM, Deng W, Jia L, Yang J, Tsurumi T, Chiang AK, et al. Role of ATM in the formation of the replication compartment during lytic replication of Epstein-Barr virus in nasopharyngeal epithelial cells. J Virol. 2015; 89:652–68.
Article24. Zebboudj A, Maroui MA, Dutrieux J, Touil-Boukoffa C, Bourouba M, Chelbi-Alix MK, et al. Sodium arsenite induces apoptosis and Epstein-Barr virus reactivation in lymphoblastoid cells. Biochimie. 2014; 107(Pt B):247–56.
Article25. Xiao L, Xiao T, Wang ZM, Cho WC, Xiao ZQ. Biomarker discovery of nasopharyngeal carcinoma by proteomics. Expert Rev Proteomics. 2014; 11:215–25.
Article26. Sun R, Wang X, Li X. Correlation analysis of nasopharyngeal carcinoma TNM staging with serum EA IgA and VCA IgA in EBV and VEGF-C and -D. Med Sci Monit. 2015; 21:2105–9.
Article27. He YQ, Xue WQ, Xu FH, Xu YF, Zhang JB, Yu HL, et al. The relationship between environmental factors and the profile of Epstein-Barr virus antibodies in the lytic and latent infection periods in healthy populations from endemic and non-endemic nasopharyngeal carcinoma areas in China. EBio-Medicine. 2018; 30:184–91.
Article28. Xu XF, Lu RQ, Xiao R, Zhou L, Zhao XM, Hu XC, et al. Rta-IgG as a biomarker for diagnosis and post treatment prognostic of nasopharyngeal carcinoma. Cancer Biomark. 2016; 16:467–76.
Article29. Cai YL, Li J, Lu AY, Zheng YM, Zhong WM, Wang W, et al. Diagnostic significance of combined detection of Epstein-Barr virus antibodies, VCA/IgA, EA/IgA, Rta/IgG and EBNA1/IgA for nasopharyngeal carcinoma. Asian Pac J Cancer Prev. 2014; 15:2001–6.
Article30. Lin JC, Wang WY, Chen KY, Wei YH, Liang WM, Jan JS, et al. Quantification of plasma Epstein-Barr virus DNA in patients with advanced nasopharyngeal carcinoma. N Engl J Med. 2004; 350:2461–70.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Anticonvulsant Hypersensitivity Syndrome Associated with Epstein-Barr Virus Reactivation
- A Case of Epstein-Barr Virus-Positive Diffuse Large B-Cell Lymphoma Occurring in Thyroid Gland
- Epstein-Barr Virus Positive Primary Lymphoepithelial Carcinoma of the Parotid Gland with Hot Uptake of the Nasopharynx at Positron Emission Tomography-Computed Tomography
- Epstein-Barr Virus-Associated Gastric Carcinoma and Specific Features of the Accompanying Immune Response
- Epstein-Barr Virus-associated Gastric Carcinoma