Korean J Physiol Pharmacol.
2021 Nov;25(6):533-543. 10.4196/kjpp.2021.25.6.533.
Triptolide improves myocardial fibrosis in rats through inhibition of nuclear factor kappa B and NLR family pyrin domain containing 3 inflammasome pathway
- Affiliations
-
- 1Department of Cardiology, The Central Hospital Affiliated to Shaoxing University, Shaoxing 312030, China
- KMID: 2521479
- DOI: http://doi.org/10.4196/kjpp.2021.25.6.533
Abstract
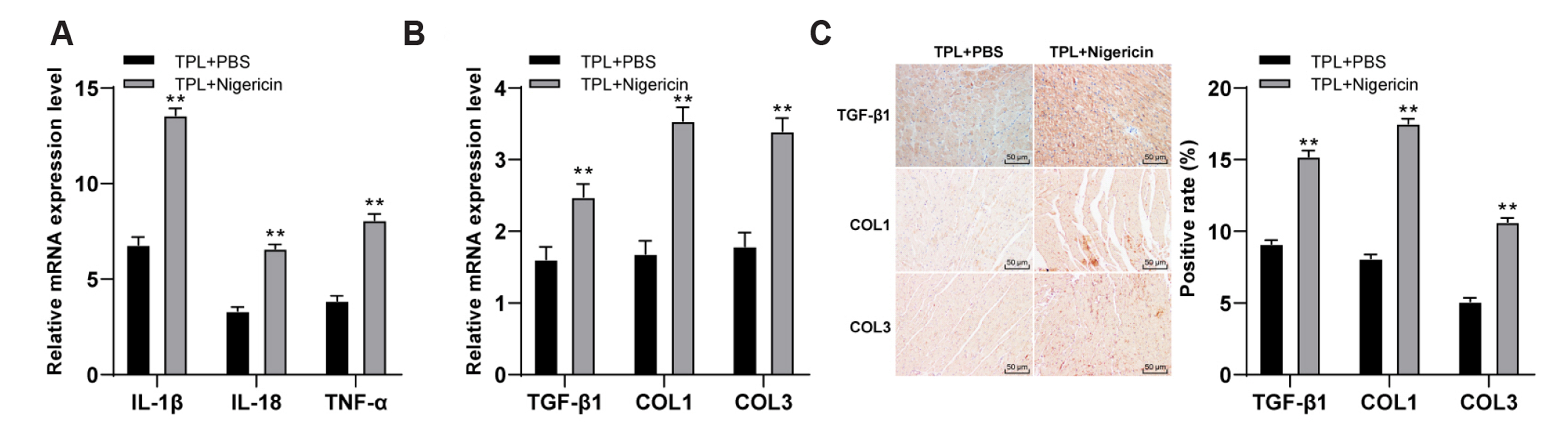
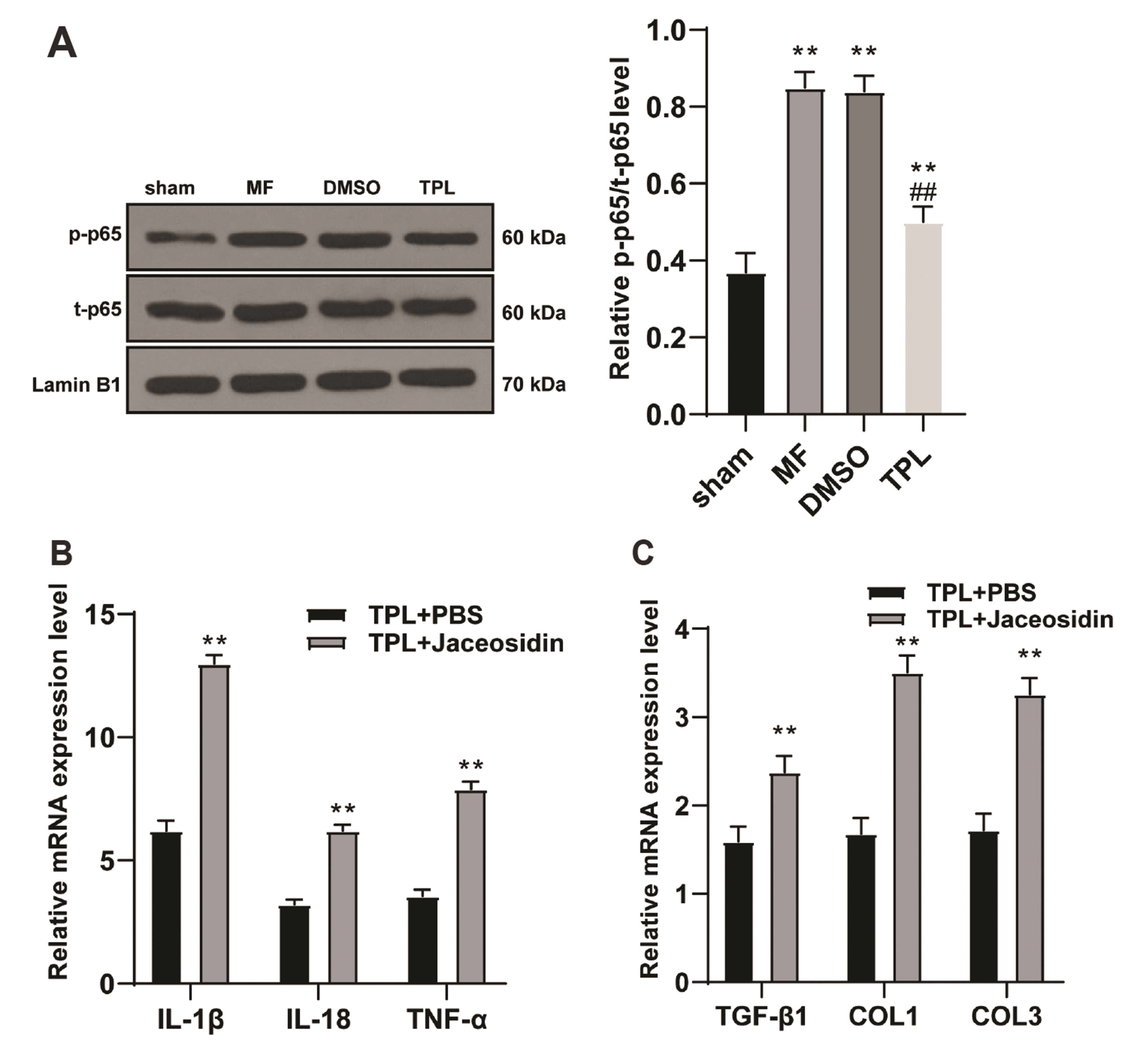
- Myocardial fibrosis (MF) is the result of persistent and repeated aggravation of myocardial ischemia and hypoxia, leading to the gradual development of heart failure of chronic ischemic heart disease. Triptolide (TPL) is identified to be involved in the treatment for MF. This study aims to explore the mechanism of TPL in the treatment of MF. The MF rat model was established, subcutaneously injected with isoproterenol and treated by subcutaneous injection of TPL. The cardiac function of each group was evaluated, including LVEF, LVFS, LVES, and LVED. The expressions of ANP, BNP, inflammatory related factors (IL-1β, IL-18, TNF-α, MCP-1, VCAM-1), NLRP3 inflammasome factors (NLRP3, ASC) and fibrosis related factors (TGF-β1, COL1, and COL3) in rats were dete cted. H&E staining and Masson staining were used to observe myocardial cell inflammation and fibrosis of rats. Western blot was used to detect the p-P65 and t-P65 levels in nucleoprotein of rat myocardial tissues. LVED and LVES of MF group were significantly upregulated, LVEF and LVFS were significantly downregulated, while TPL treatment reversed these trends; TPL treatment downregulated the tissue injury and improved the pathological damage of MF rats. TPL treatment downregulated the levels of inflammatory factors and fibrosis factors, and inhibited the activation of NLRP3 inflammasome. Activation of NLRP3 inflammasome or NF-κB pathway reversed the effect of TPL on MF. Collectively, TPL inhibited the activation of NLRP3 inflammasome by inhibiting NF-κB pathway, and improved MF in MF rats.
Keyword
Figure
Cited by 1 articles
-
The role of discoid domain receptor 1 on renal tubular epithelial pyroptosis in diabetic nephropathy
Weichen Zhao, Chunyuan He, Junjie Jiang, Zongbiao Zhao, Hongzhong Yuan, Facai Wang, Bingxiang Shen
Korean J Physiol Pharmacol. 2022;26(6):427-438. doi: 10.4196/kjpp.2022.26.6.427.
Reference
-
1. Gyöngyösi M, Winkler J, Ramos I, Do QT, Firat H, McDonald K, González A, Thum T, Díez J, Jaisser F, Pizard A, Zannad F. 2017; Myocardial fibrosis: biomedical research from bench to bedside. Eur J Heart Fail. 19:177–191. DOI: 10.1002/ejhf.696. PMID: 28157267. PMCID: PMC5299507.
Article2. van de Schoor FR, Aengevaeren VL, Hopman MT, Oxborough DL, George KP, Thompson PD, Eijsvogels TM. 2016; Myocardial fibrosis in athletes. Mayo Clin Proc. 91:1617–1631. DOI: 10.1016/j.mayocp.2016.07.012. PMID: 27720455.
Article3. Everett RJ, Stirrat CG, Semple SI, Newby DE, Dweck MR, Mirsadraee S. 2016; Assessment of myocardial fibrosis with T1 mapping MRI. Clin Radiol. 71:768–778. DOI: 10.1016/j.crad.2016.02.013. PMID: 27005015.
Article4. Shinde AV, Frangogiannis NG. 2014; Fibroblasts in myocardial infarction: a role in inflammation and repair. J Mol Cell Cardiol. 70:74–82. DOI: 10.1016/j.yjmcc.2013.11.015. PMID: 24321195. PMCID: PMC3995820.
Article5. Marques MD, Nauffal V, Ambale-Venkatesh B, Vasconcellos HD, Wu C, Bahrami H, Tracy RP, Cushman M, Bluemke DA, Lima JAC. 2018; Association between inflammatory markers and myocardial fibrosis. Hypertension. 72:902–908. DOI: 10.1161/HYPERTENSIONAHA.118.11463. PMID: 30354713. PMCID: PMC6205739.
Article6. López B, González A, Ravassa S, Beaumont J, Moreno MU, San José G, Querejeta R, Díez J. 2015; Circulating biomarkers of myocardial fibrosis: the need for a reappraisal. J Am Coll Cardiol. 65:2449–2456. DOI: 10.1016/j.jacc.2015.04.026. PMID: 26046739.7. Wei YM, Wang YH, Xue HQ, Luan ZH, Liu BW, Ren JH. 2019; Triptolide, a potential autophagy modulator. Chin J Integr Med. 25:233–240. DOI: 10.1007/s11655-018-2847-z. PMID: 30178091.
Article8. Noel P, Von Hoff DD, Saluja AK, Velagapudi M, Borazanci E, Han H. 2019; Triptolide and its derivatives as cancer therapies. Trends Pharmacol Sci. 40:327–341. DOI: 10.1016/j.tips.2019.03.002. PMID: 30975442.
Article9. Viegas JSR, Praça FG, Kravicz M, Bentley MVLB. 2020; Therapeutic applications and delivery systems for triptolide. Drug Deliv Transl Res. 10:1584–1600. DOI: 10.1007/s13346-020-00827-z. PMID: 32789808.
Article10. Yuan K, Li X, Lu Q, Zhu Q, Jiang H, Wang T, Huang G, Xu A. 2019; Application and mechanisms of triptolide in the treatment of inflammatory diseases-a review. Front Pharmacol. 10:1469. DOI: 10.3389/fphar.2019.01469. PMID: 31866868. PMCID: PMC6908995.
Article11. Pan XC, Liu Y, Cen YY, Xiong YL, Li JM, Ding YY, Tong YF, Liu T, Chen XH, Zhang HG. 2019; Dual role of triptolide in interrupting the NLRP3 inflammasome pathway to attenuate cardiac fibrosis. Int J Mol Sci. 20:360. DOI: 10.3390/ijms20020360. PMID: 30654511. PMCID: PMC6359320.
Article12. Kong P, Christia P, Frangogiannis NG. 2014; The pathogenesis of cardiac fibrosis. Cell Mol Life Sci. 71:549–574. DOI: 10.1007/s00018-013-1349-6. PMID: 23649149. PMCID: PMC3769482.
Article13. Ralston JC, Lyons CL, Kennedy EB, Kirwan AM, Roche HM. 2017; Fatty acids and NLRP3 inflammasome-mediated inflammation in metabolic tissues. Annu Rev Nutr. 37:77–102. DOI: 10.1146/annurev-nutr-071816-064836. PMID: 28826373.
Article14. Gao R, Shi H, Chang S, Gao Y, Li X, Lv C, Yang H, Xiang H, Yang J, Xu L, Tang Y. 2019; The selective NLRP3-inflammasome inhibitor MCC950 reduces myocardial fibrosis and improves cardiac remodeling in a mouse model of myocardial infarction. Int Immunopharmacol. 74:105575. DOI: 10.1016/j.intimp.2019.04.022. PMID: 31299609.
Article15. Wu W, Liu BH, Wan YG, Sun W, Liu YL, Wang WW, Fang QJ, Tu Y, Yee HY, Yuan CC, Wan ZY. 2019; [Triptolide inhibits NLRP3 inflammasome activation and ameliorates podocyte epithelial-mesenchymal transition induced by high glucose]. Zhongguo Zhong Yao Za Zhi. 44:5457–5464. Chinese. DOI: 10.19540/j.cnki.cjcmm.20191114.401. PMID: 32237395.16. Qian K, Zhang L, Shi K. 2019; Triptolide prevents osteoarthritis via inhibiting hsa-miR-20b. Inflammopharmacology. 27:109–119. DOI: 10.1007/s10787-018-0509-6. PMID: 29974310.
Article17. Guo X, Xue M, Li CJ, Yang W, Wang SS, Ma ZJ, Zhang XN, Wang XY, Zhao R, Chang BC, Chen LM. 2016; Protective effects of triptolide on TLR4 mediated autoimmune and inflammatory response induced myocardial fibrosis in diabetic cardiomyopathy. J Ethnopharmacol. 193:333–344. DOI: 10.1016/j.jep.2016.08.029. PMID: 27558948.
Article18. Wan Y, Xu L, Wang Y, Tuerdi N, Ye M, Qi R. 2018; Preventive effects of astragaloside IV and its active sapogenin cycloastragenol on cardiac fibrosis of mice by inhibiting the NLRP3 inflammasome. Eur J Pharmacol. 833:545–554. DOI: 10.1016/j.ejphar.2018.06.016. PMID: 29913124.
Article19. Han C, Yang Y, Guan Q, Zhang X, Shen H, Sheng Y, Wang J, Zhou X, Li W, Guo L, Jiao Q. 2020; New mechanism of nerve injury in Alzheimer's disease: β-amyloid-induced neuronal pyroptosis. J Cell Mol Med. 24:8078–8090. DOI: 10.1111/jcmm.15439. PMID: 32521573. PMCID: PMC7348172.
Article20. Huang XL, Wei XC, Guo LQ, Zhao L, Chen XH, Cui YD, Yuan J, Chen DF, Zhang J. 2019; The therapeutic effects of Jaceosidin on lipopolysaccharide-induced acute lung injury in mice. J Pharmacol Sci. 140:228–235. DOI: 10.1016/j.jphs.2019.07.004. PMID: 31358372.
Article21. Zatroch KK, Knight CG, Reimer JN, Pang DS. 2017; Refinement of intraperitoneal injection of sodium pentobarbital for euthanasia in laboratory rats (Rattus norvegicus). BMC Vet Res. 13:60. DOI: 10.1186/s12917-017-0982-y. PMID: 28222732. PMCID: PMC5320784.
Article22. Špiranec Spes K, Chen W, Krebes L, Völker K, Abeßer M, Eder Negrin P, Cellini A, Nickel A, Nikolaev VO, Hofmann F, Schuh K, Schweda F, Kuhn M. 2020; Heart-microcirculation connection: effects of ANP (atrial natriuretic peptide) on pericytes participate in the acute and chronic regulation of arterial blood pressure. Hypertension. 76:1637–1648. DOI: 10.1161/HYPERTENSIONAHA.120.15772. PMID: 32951468.23. Li X, Zhao D, Guo Z, Li T, Qili M, Xu B, Qian M, Liang H, E X, Chege Gitau S, Wang L, Huangfu L, Wu Q, Xu C, Shan H. 2016; Overexpression of SerpinE2/protease nexin-1 contribute to pathological cardiac fibrosis via increasing collagen deposition. Sci Rep. 6:37635. DOI: 10.1038/srep37635. PMID: 27876880. PMCID: PMC5120308.
Article24. Unudurthi SD, Nassal DM, Patel NJ, Thomas E, Yu J, Pierson CG, Bansal SS, Mohler PJ, Hund TJ. 2020; Fibroblast growth factor-inducible 14 mediates macrophage infiltration in heart to promote pressure overload-induced cardiac dysfunction. Life Sci. 247:117440. DOI: 10.1016/j.lfs.2020.117440. PMID: 32070706. PMCID: PMC7433891.
Article25. Zhou Z, Miao Z, Luo A, Zhu D, Lu Y, Li P, Feng X, Tan W, Wang F. 2020; Identifying a marked inflammation mediated cardiac dysfunction during the development of arthritis in collagen-induced arthritis mice. Clin Exp Rheumatol. 38:203–211. PMID: 31140393.26. Kong DH, Kim YK, Kim MR, Jang JH, Lee S. 2018; Emerging roles of vascular cell adhesion molecule-1 (VCAM-1) in immunological disorders and cancer. Int J Mol Sci. 19:1057. DOI: 10.3390/ijms19041057. PMID: 29614819. PMCID: PMC5979609.
Article27. Yoshimura T. 2017; The production of monocyte chemoattractant protein-1 (MCP-1)/CCL2 in tumor microenvironments. Cytokine. 98:71–78. DOI: 10.1016/j.cyto.2017.02.001. PMID: 28189389.
Article28. He XF, Zeng YX, Li G, Feng YK, Wu C, Liang FY, Zhang Y, Lan Y, Xu GQ, Pei Z. 2020; Extracellular ASC exacerbated the recurrent ischemic stroke in an NLRP3-dependent manner. J Cereb Blood Flow Metab. 40:1048–1060. DOI: 10.1177/0271678X19856226. PMID: 31216943. PMCID: PMC7181081.
Article29. Qu S, Wang W, Li D, Li S, Zhang L, Fu Y, Zhang N. 2017; Mangiferin inhibits mastitis induced by LPS via suppressing NF-ĸB and NLRP3 signaling pathways. Int Immunopharmacol. 43:85–90. DOI: 10.1016/j.intimp.2016.11.036. PMID: 27984712.
Article30. Viswanadha VP, Dhivya V, Beeraka NM, Huang CY, Gavryushova LV, Minyaeva NN, Chubarev VN, Mikhaleva LM, Tarasov VV, Aliev G. 2020; The protective effect of piperine against isoproterenol-induced inflammation in experimental models of myocardial toxicity. Eur J Pharmacol. 885:173524. DOI: 10.1016/j.ejphar.2020.173524. PMID: 32882215.
Article31. Talman V, Ruskoaho H. 2016; Cardiac fibrosis in myocardial infarction-from repair and remodeling to regeneration. Cell Tissue Res. 365:563–581. DOI: 10.1007/s00441-016-2431-9. PMID: 27324127. PMCID: PMC5010608.
Article32. Ravassa S, González A, Bayés-Genís A, Lupón J, Díez J. 2020; Myocardial interstitial fibrosis in the era of precision medicine. Biomarker-based phenotyping for a personalized treatment. Rev Esp Cardiol (Engl Ed). 73:248–254. DOI: 10.1016/j.rec.2019.09.010. PMID: 31759935.
Article33. Jiang W, Chen M, Xiao C, Yang W, Qin Q, Tan Q, Liang Z, Liao X, Mao A, Wei C. 2019; Triptolide suppresses growth of breast cancer by targeting HMGB1 in vitro and in vivo. Biol Pharm Bull. 42:892–899. DOI: 10.1248/bpb.b18-00818. PMID: 30956264.34. Kim ST, Kim SY, Lee J, Kim K, Park SH, Park YS, Lim HY, Kang WK, Park JO. 2018; Triptolide as a novel agent in pancreatic cancer: the validation using patient derived pancreatic tumor cell line. BMC Cancer. 18:1103. DOI: 10.1186/s12885-018-4995-0. PMID: 30419860. PMCID: PMC6233492.
Article35. Reno TA, Kim JY, Raz DJ. 2015; Triptolide inhibits lung cancer cell migration, invasion, and metastasis. Ann Thorac Surg. 100:1817–1824. DOI: 10.1016/j.athoracsur.2015.05.074. PMID: 26298168. PMCID: PMC4630145.
Article36. Banerjee S, Saluja A. 2015; Minnelide, a novel drug for pancreatic and liver cancer. Pancreatology. 15(4 Suppl):S39–S43. DOI: 10.1016/j.pan.2015.05.472. PMID: 26122306. PMCID: PMC4515388.
Article37. Chugh R, Sangwan V, Patil SP, Dudeja V, Dawra RK, Banerjee S, Schumacher RJ, Blazar BR, Georg GI, Vickers SM, Saluja AK. 2012; A preclinical evaluation of Minnelide as a therapeutic agent against pancreatic cancer. Sci Transl Med. 4:156ra139. DOI: 10.1126/scitranslmed.3004334. PMID: 23076356. PMCID: PMC3656604.
Article38. Giri B, Gupta VK, Yaffe B, Modi S, Roy P, Sethi V, Lavania SP, Vickers SM, Dudeja V, Banerjee S, Watts J, Saluja A. 2019; Pre-clinical evaluation of Minnelide as a therapy for acute myeloid leukemia. J Transl Med. 17:163. DOI: 10.1186/s12967-019-1901-8. PMID: 31109340. PMCID: PMC6528210.
Article39. Ramakrishnan V, de Haydu C, Wilkinson P, Hooda U, Giri B, Oleas JM, Rive V, Roy S, Dudeja V, Slomovitch B, Saluja A, Ramakrishnan S. 2021; Minnelide, a prodrug, inhibits cervical cancer growth by blocking HPV-induced changes in p53 and pRb. Am J Cancer Res. 11:2202–2214. PMID: 34094678. PMCID: PMC8167699.40. Rivard C, Geller M, Schnettler E, Saluja M, Vogel RI, Saluja A, Ramakrishnan S. 2014; Inhibition of epithelial ovarian cancer by Minnelide, a water-soluble pro-drug. Gynecol Oncol. 135:318–324. DOI: 10.1016/j.ygyno.2014.08.031. PMID: 25172764. PMCID: PMC4582668.
Article41. Ding YY, Li JM, Guo FJ, Liu Y, Tong YF, Pan XC, Lu XL, Ye W, Chen XH, Zhang HG. 2016; Triptolide upregulates myocardial forkhead helix transcription factor p3 expression and attenuates cardiac hypertrophy. Front Pharmacol. 7:471. DOI: 10.3389/fphar.2016.00471. PMID: 27965581. PMCID: PMC5127789.
Article42. Li W, Gong K, Ding Y, Chaurasiya B, Ni Y, Wu Y, Zhao P, Shen Y, Zhang Z, Webster TJ. 2019; Effects of triptolide and methotrexate nanosuspensions on left ventricular remodeling in autoimmune myocarditis rats. Int J Nanomedicine. 14:851–863. DOI: 10.2147/IJN.S191267. PMID: 30774338. PMCID: PMC6361222.43. Liu M, Chen J, Huang Y, Ke J, Li L, Huang D, Wu W. 2015; Triptolide alleviates isoprenaline-induced cardiac remodeling in rats via TGF-β1/Smad3 and p38 MAPK signaling pathway. Pharmazie. 70:244–250. PMID: 26012254.44. Ge T, Qin H, Wang X, Yang SS, Guo L, Han W, Chang HY. 2014; Effects of thoracic epidural anesthesia on cardiac function and myocardial cell apoptosis in isoproterenol-induced chronic heart failure rats. J. Interv Cardiol. 27:446–455. DOI: 10.1111/joic.12147. PMID: 25267251.45. Liang Z, Leo S, Wen H, Ouyang M, Jiang W, Yang K. 2015; Triptolide improves systolic function and myocardial energy metabolism of diabetic cardiomyopathy in streptozotocin-induced diabetic rats. BMC Cardiovasc Disord. 15:42. DOI: 10.1186/s12872-015-0030-4. PMID: 25967112. PMCID: PMC4431461.
Article46. Liu Y, Liu PP, Liu L, Zheng XS, Zheng H, Yang CC, Luobu CR, Liu Y. 2018; Triptolide inhibits TGF-β-induced matrix contraction and fibronectin production mediated by human Tenon fibroblasts. Int J Ophthalmol. 11:1108–1113. DOI: 10.18240/ijo.2018.07.06. PMID: 30046525. PMCID: PMC6048341.
Article47. Li XY, Wang SS, Han Z, Han F, Chang YP, Yang Y, Xue M, Sun B, Chen LM. 2017; Triptolide restores autophagy to alleviate diabetic renal fibrosis through the miR-141-3p/PTEN/Akt/mTOR pathway. Mol Ther Nucleic Acids. 9:48–56. DOI: 10.1016/j.omtn.2017.08.011. PMID: 29246323. PMCID: PMC5602517.
Article48. Fang YY, Wan L, Dong WZ, Wen JT, Liu J. 2019; [Effect of triptolide in improving platelet activation in patients with ankylosing spondylitis by regulating VEGFA,SDF-1,CXCR4 pathway]. Zhongguo Zhong Yao Za Zhi. 44:3520–3525. Chinese. DOI: 10.19540/j.cnki.cjcmm.20181113.001. PMID: 31602917.49. Song C, Wang Y, Cui L, Yan F, Shen S. 2019; Triptolide attenuates lipopolysaccharide-induced inflammatory responses in human endothelial cells: involvement of NF-κB pathway. BMC Complement Altern Med. 19:198. DOI: 10.1186/s12906-019-2616-3. PMID: 31375092. PMCID: PMC6679459.
Article50. Martínez GJ, Celermajer DS, Patel S. 2018; The NLRP3 inflammasome and the emerging role of colchicine to inhibit atherosclerosis-associated inflammation. Atherosclerosis. 269:262–271. DOI: 10.1016/j.atherosclerosis.2017.12.027. PMID: 29352570.
Article51. Li R, Lu K, Wang Y, Chen M, Zhang F, Shen H, Yao D, Gong K, Zhang Z. 2017; Triptolide attenuates pressure overload-induced myocardial remodeling in mice via the inhibition of NLRP3 inflammasome expression. Biochem Biophys Res Commun. 485:69–75. DOI: 10.1016/j.bbrc.2017.02.021. PMID: 28202417.
Article52. He L, Peng X, Liu G, Tang C, Liu H, Liu F, Zhou H, Peng Y. 2015; Anti-inflammatory effects of triptolide on IgA nephropathy in rats. Immunopharmacol Immunotoxicol. 37:421–427. DOI: 10.3109/08923973.2015.1080265. PMID: 26466641.
Article53. Wang L, Zhang L, Hou Q, Zhu X, Chen Z, Liu Z. 2018; Triptolide attenuates proteinuria and podocyte apoptosis via inhibition of NF-κB/GADD45B. Sci Rep. 8:10843. DOI: 10.1038/s41598-018-29203-1. PMID: 30022148. PMCID: PMC6052061.
Article54. Xie S, Deng W, Chen J, Wu QQ, Li H, Wang J, Wei L, Liu C, Duan M, Cai Z, Xie Q, Hu T, Zeng X, Tang Q. 2020; Andrographolide protects against adverse cardiac remodeling after myocardial infarction through enhancing Nrf2 signaling pathway. Int J Biol Sci. 16:12–26. DOI: 10.7150/ijbs.37269. PMID: 31892842. PMCID: PMC6930369.
Article55. Xi C, Peng S, Wu Z, Zhou Q, Zhou J. 2017; Toxicity of triptolide and the molecular mechanisms involved. Biomed Pharmacother. 90:531–541. DOI: 10.1016/j.biopha.2017.04.003. PMID: 28402922.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pyrin Domain (PYD)-containing Inflammasome in Innate Immunity
- Extracellular Acidification Augments NLRP3-Mediated Inflammasome Signaling in Macrophages
- The role of discoid domain receptor 1 on renal tubular epithelial pyroptosis in diabetic nephropathy
- PF-04620110, a Potent Antidiabetic Agent, Suppresses Fatty Acid-Induced NLRP3 Inflammasome Activation in Macrophages
- A20 Protects Against Arthritis by Regulation of the NLRP3 Inflammasome