J Korean Med Sci.
2021 Oct;36(40):e286. 10.3346/jkms.2021.36.e286.
Myocarditis-induced Sudden Death after BNT162b2 mRNA COVID-19 Vaccination in Korea: Case Report Focusing on Histopathological Findings
- Affiliations
-
- 1Department of Forensic Medicine, Defense Institute of Forensic Science, Criminal Investigation Command, Ministry of National Defense, Seoul, Korea
- 2Department of Pathology, Incheon Sejong Hospital, Incheon, Korea
- KMID: 2521432
- DOI: http://doi.org/10.3346/jkms.2021.36.e286
Abstract
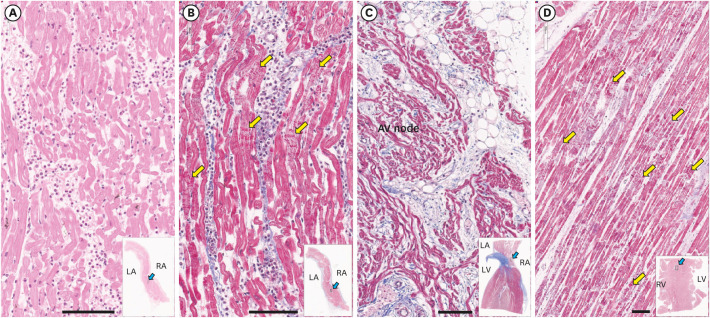
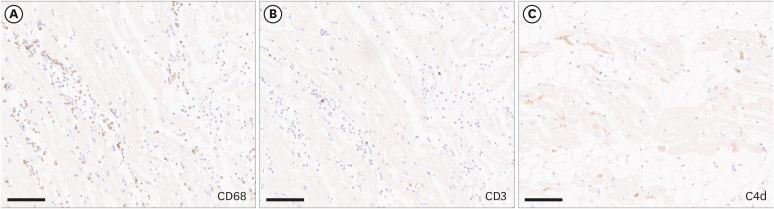
- We present autopsy findings of a 22-year-old man who developed chest pain 5 days after the first dose of the BNT162b2 mRNA vaccine and died 7 hours later. Histological examination of the heart revealed isolated atrial myocarditis, with neutrophil and histiocyte predominance. Immunohistochemical C4d staining revealed scattered single-cell necrosis of myocytes which was not accompanied by inflammatory infiltrates. Extensive contraction band necrosis was observed in the atria and ventricles. There was no evidence of microthrombosis or infection in the heart and other organs. The primary cause of death was determined to be myocarditis, causally-associated with the BNT162b2 vaccine.
Keyword
Figure
Cited by 1 articles
-
A Case Report for Acute Myopericarditis After NVX-CoV2373 (Novavax®) COVID-19 Vaccination
Hyung Yoon Kim, Jae Yeong Cho, Hyun Ju Yoon, Yoo-Duk Choi, Youngkeun Ahn, Myung Ho Jeong, Jeong Gwan Cho, Kye Hun Kim
J Korean Med Sci. 2022;37(34):e265. doi: 10.3346/jkms.2022.37.e265.
Reference
-
1. Oliver SE, Gargano JW, Marin M, Wallace M, Curran KG, Chamberland M, et al. The advisory committee on immunization practices' interim recommendation for use of Pfizer-BioNTech COVID-19 vaccine - United States, December 2020. MMWR Morb Mortal Wkly Rep. 2020; 69(50):1922–1924. PMID: 33332292.
Article2. Oliver SE, Gargano JW, Marin M, Wallace M, Curran KG, Chamberland M, et al. The advisory committee on immunization practices' interim recommendation for use of Moderna COVID-19 vaccine - United States, December 2020. MMWR Morb Mortal Wkly Rep. 2021; 69(5152):1653–1656. PMID: 33382675.
Article3. Ammirati E, Cavalotti C, Milazzo A, Pedrotti P, Soriano F, Schroeder JW, et al. Temporal relation between second dose BNT162b2 mRNA Covid-19 vaccine and cardiac involvement in a patient with previous SARS-COV-2 infection. Int J Cardiol Heart Vasc. Forthcoming. 2021; DOI: 10.1016/j.ijcha.2021.100778.
Article4. Kim HW, Jenista ER, Wendell DC, Azevedo CF, Campbell MJ, Darty SN, et al. Patients with acute myocarditis following mRNA COVID-19 vaccination. JAMA Cardiol. Forthcoming. 2021; DOI: 10.1001/jamacardio.2021.2828.
Article5. Larson KF, Ammirati E, Adler ED, Cooper LT Jr, Hong KN, Saponara G, et al. Myocarditis after BNT162b2 and mRNA-1273 vaccination. Circulation. 2021; 144(6):506–508. PMID: 34133884.
Article6. Mansour J, Short RG, Bhalla S, Woodard PK, Verma A, Robinson X, et al. Acute myocarditis after a second dose of the mRNA COVID-19 vaccine: a report of two cases. Clin Imaging. 2021; 78:247–249. PMID: 34166884.
Article7. Marshall M, Ferguson ID, Lewis P, Jaggi P, Gagliardo C, Collins JS, et al. Symptomatic acute myocarditis in seven adolescents following Pfizer-BioNTech COVID-19 vaccination. Pediatrics. 2021; 148(3):e2021052478. PMID: 34088762.
Article8. Montgomery J, Ryan M, Engler R, Hoffman D, McClenathan B, Collins L, et al. Myocarditis following immunization with mRNA COVID-19 vaccines in members of the US military. JAMA Cardiol. Forthcoming. 2021; DOI: 10.1001/jamacardio.2021.2833.
Article9. Rosner CM, Genovese L, Tehrani BN, Atkins M, Bakhshi H, Chaudhri S, et al. Myocarditis temporally associated with COVID-19 vaccination. Circulation. 2021; 144(6):502–505. PMID: 34133885.
Article10. Verma AK, Lavine KJ, Lin CY. Myocarditis after Covid-19 mRNA vaccination. N Engl J Med. 2021; 385(14):1332–1334. PMID: 34407340.
Article11. Snapiri O, Rosenberg Danziger C, Shirman N, Weissbach A, Lowenthal A, Ayalon I, et al. Transient cardiac injury in adolescents receiving the BNT162b2 mRNA COVID-19 vaccine. Pediatr Infect Dis J. 2021; 40(10):e360–e363. PMID: 34077949.
Article12. Kim IC, Kim H, Lee HJ, Kim JY, Kim JY. Cardiac imaging of acute myocarditis following COVID-19 mRNA vaccination. J Korean Med Sci. 2021; 36(2):e229. PMID: 34402228.
Article13. Aretz HT, Billingham ME, Edwards WD, Factor SM, Fallon JT, Fenoglio JJ Jr, et al. Myocarditis. A histopathologic definition and classification. Am J Cardiovasc Pathol. 1987; 1(1):3–14. PMID: 3455232.14. Leone O, Pieroni M, Rapezzi C, Olivotto I. The spectrum of myocarditis: from pathology to the clinics. Virchows Arch. 2019; 475(3):279–301. PMID: 31297595.
Article15. Tse R, Garland J, Kesha K, Triggs Y, Modahl L, Milne D, et al. A rare case of isolated atrial myocarditis causing death with no post mortem computed tomography scan correlation. Am J Forensic Med Pathol. 2018; 39(2):123–125. PMID: 29120873.
Article16. Duffy M, O'Connor K, Milne D, Ondruschka B, Tse R, Garland J. Isolated atrial neutrophilic myocarditis: a rare cause of death and potential “Blind Spot” for postmortem computed tomography and postmortem examination. Am J Forensic Med Pathol. Forthcoming. 2021; DOI: 10.1097/PAF.0000000000000684.17. Halushka MK, Vander Heide RS. Myocarditis is rare in COVID-19 autopsies: cardiovascular findings across 277 postmortem examinations. Cardiovasc Pathol. 2021; 50:107300. PMID: 33132119.
Article18. Jum'ah H, Loeffler A, Tomashefski JF Jr. Histopathological findings in the hearts of COVID-19 autopsies: a letter to cardiovascular pathology journal editor in response to Halushka et al. 2020. Cardiovasc Pathol. 2021; 52:107333. PMID: 33741530.19. Fox SE, Akmatbekov A, Harbert JL, Li G, Quincy Brown J, Vander Heide RS. Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans. Lancet Respir Med. 2020; 8(7):681–686. PMID: 32473124.
Article20. Baroldi G, Mittleman RE, Parolini M, Silver MD, Fineschi V. Myocardial contraction bands. Definition, quantification and significance in forensic pathology. Int J Legal Med. 2001; 115(3):142–151. PMID: 11775016.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Myocarditis Presenting With a Hyperechoic Nodule After the First Dose of COVID-19 mRNA Vaccine
- New-onset Refractory Status Epilepticus after BNT162b2 mRNA COVID-19 Vaccination
- Sudden Cardiac Death Caused by Cardiac Small Vessel Vasculitis after COVID-19 Vaccination (BNT162b2 nCov-19): A Case Report
- Cardiac Imaging of Acute Myocarditis Following COVID-19 mRNA Vaccination
- Adult-onset Still’s Disease after BNT162b2 mRNA COVID-19 Vaccine