J Stroke.
2021 Sep;23(3):358-366. 10.5853/jos.2021.00724.
Mechanical Thrombectomy in Patients with a Large Ischemic Volume at Presentation: Systematic Review and Meta-Analysis
- Affiliations
-
- 1Department of Neuroradiology, GHU Paris, Sainte Anne Hospital Pscyhiatry and Neurosciences Institute (IPNP), UMR_S1266, INSERM, University of Paris, Tours, France
- 2Department of Neuroradiology, University Hospital of Tours, Tours, France
- 3Department of Neuroradiology, University Hospital of Marseille La Timone, Marseille, France
- 4Institute of Diagnostic, Interventional and Pediatric Radiology and Institute of Diagnostic and Interventional Neuroradiology, University Hospital Bern, Inselspital, University of Bern, Bern, Switzerland
- 5Diagnostic and Interventional Neuroradiology, University Hospital of Lille, Lille, France
- 6Neuroradiology Department and Stroke Unit, University Hospital of Lille, Lille, France
- 7Department of Cerebrovascular Medicine National Cerebral and Cardiovascular Center, Suita, Japan
- 8Department of Diagnostic and Interventional Neuroradiology, Foch Hospital, Suresnes, France
- 9Neuroradiology Department, University Hospital of Gui de Chauliac, Montpellier, France
- 10Department of Interventional Neuroradiology, Foch Hospital, Suresnes, France
- KMID: 2520913
- DOI: http://doi.org/10.5853/jos.2021.00724
Abstract
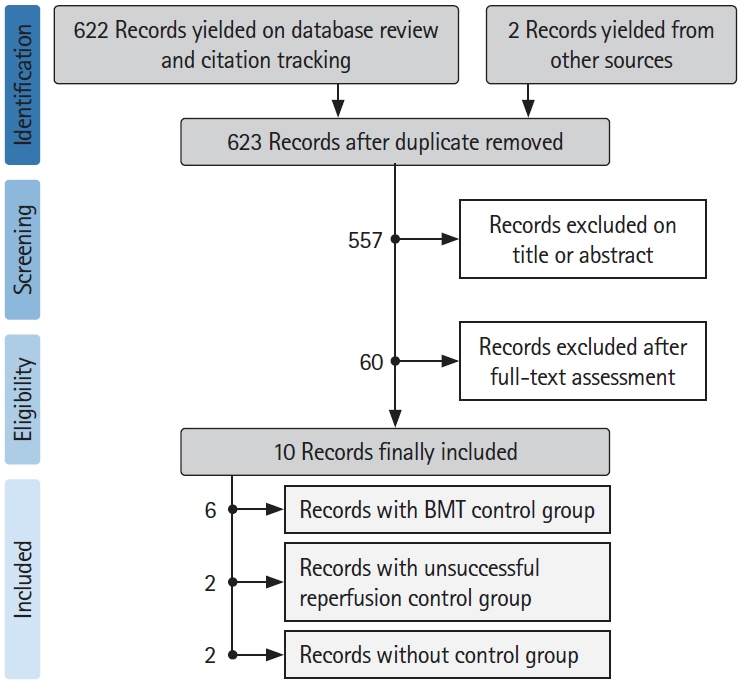
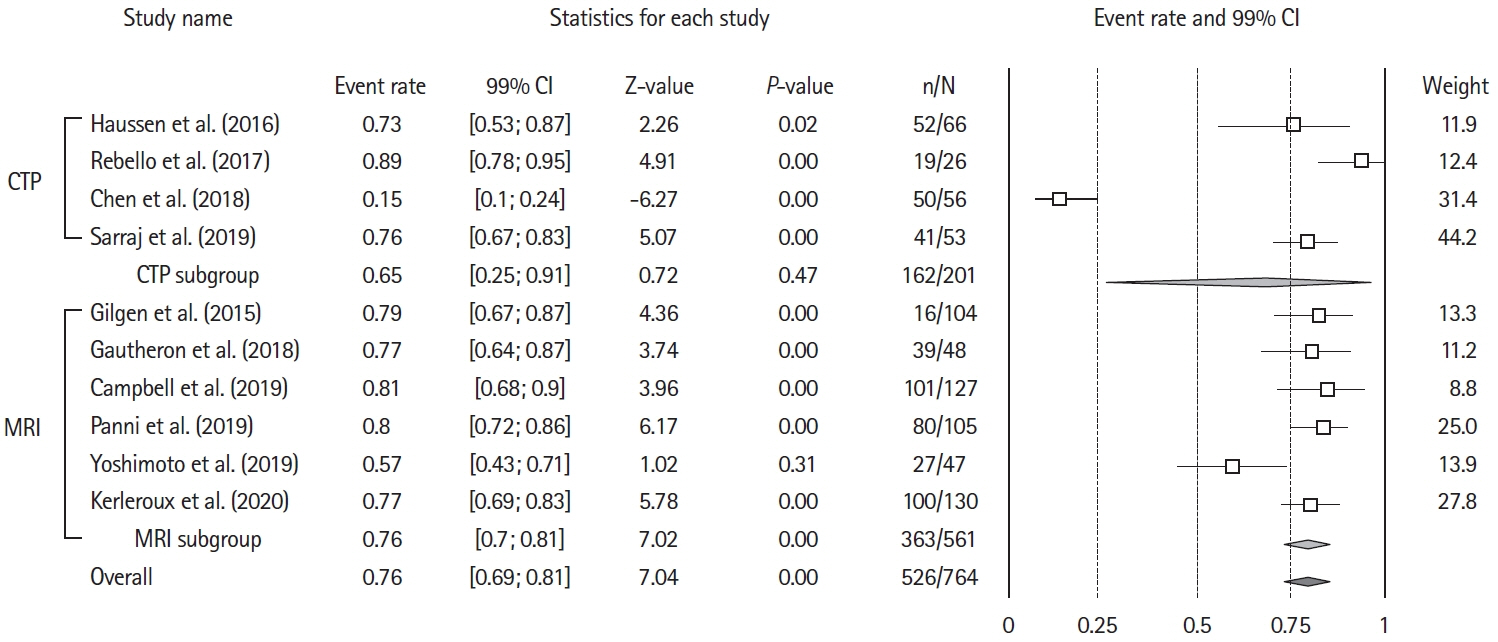
- The benefits of mechanical thrombectomy (MT) for patients with acute ischemic stroke (AIS) and a large ischemic core (LIC) at presentation are uncertain. We aimed to obtain up-to-date aggregate estimates of the outcomes following MT in patients with volumetrically assessed LIC. We conducted a Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA)-conformed, PROSPERO-registered, systematic review and meta-analysis of studies that included patients with AIS and a baseline LIC treated with MT, reported ischemic core volume quantitatively, and included patients with a LIC defined as a core volume ≥50 mL. The search was restricted to studies published between January 2015 and June 2020. Random-effects-meta-analysis was used to assess the effect of MT on 90-day unfavorable outcome (i.e., modified Rankin Scale [mRS] 3–6), mortality, and symptomatic intracranial hemorrhage (sICH) occurrence. Sensitivity analyses were performed for imaging-modality (computed tomography-perfusion or magnetic resonance-diffusion weighted imaging) and LIC-definition (≥50 or ≥70 mL). We analyzed 10 studies (954 patients), including six (682 patients) with a control group, allowing to compare 332 patients with MT to 350 who received best-medical-management alone. Overall, after MT the rate of patients with mRS 3–6 at 90 days was 74% (99% confidence interval [CI], 67 to 84; Z-value=7.04; I2=92.3%) and the rate of 90-day mortality was 36% (99% CI, 33 to 40; Z-value=–7.07; I2=74.5). Receiving MT was associated with a significant decrease in mRS 3–6 odds ratio (OR) 0.19 (99% CI, 0.11 to 0.33; P<0.01; Z-value=–5.92; I2=62.56) and in mortality OR 0.60 (99% CI, 0.34 to 1.06; P=0.02; Z-value=–2.30; I2=58.72). Treatment group did not influence the proportion of patients experiencing sICH, OR 0.96 (99% CI, 0.2 to 1.49; P=0.54; Z-value=–0.63; I2=64.74). Neither imaging modality for core assessment, nor LIC definition influenced the aggregated outcomes. Using aggregate estimates, MT appeared to decrease the risk of unfavorable functional outcome in patients with a LIC assessed volumetrically at baseline.
Keyword
Figure
Cited by 1 articles
-
Endovascular Thrombectomy for Large Ischemic Strokes: A Living Systematic Review and Meta-Analysis of Randomized Trials
Rami Z. Morsi, Mohamed Elfil, Hazem S. Ghaith, Mohammad Aladawi, Ahmad Elmashad, Sachin Kothari, Harsh Desai, Shyam Prabhakaran, Fawaz Al-Mufti, Tareq Kass-Hout
J Stroke. 2023;25(2):214-222. doi: 10.5853/jos.2023.00752.
Reference
-
References
1. Turc G, Bhogal P, Fischer U, Khatri P, Lobotesis K, Mazighi M, et al. European Stroke Organisation (ESO): European Society for minimally invasive neurological therapy (ESMINT) guidelines on mechanical thrombectomy in acute ischemic stroke. J Neurointerv Surg. 2019; 11:535–538.
Article2. Albers GW. Endovascular thrombectomy in patients with large infarctions: reasons for restraint. Lancet Neurol. 2018; 17:836–837.
Article3. Kerleroux B, Janot K, Dargazanli C, Daly-Eraya D, Ben-Hassen W, Zhu F, et al. Perfusion imaging to select patients with large ischemic core for mechanical thrombectomy. J Stroke. 2020; 22:225–233.
Article4. Cagnazzo F, Derraz I, Dargazanli C, Lefevre PH, Gascou G, Riquelme C, et al. Mechanical thrombectomy in patients with acute ischemic stroke and ASPECTS ≤6: a meta-analysis. J Neurointerv Surg. 2020; 12:350–355.
Article5. Khoury N, Dargazanli C, Zuber K, Smajda S, Bitar M, Boulouis G, et al. Diffusion-weighted-imaging infarct volume measurement tools show discrepancies leading to diverging thrombectomy decisions. J Neuroradiol. 2021; 48:305–310.
Article6. Gilgen MD, Klimek D, Liesirova KT, Meisterernst J, KlingerGratz PP, Schroth G, et al. Younger stroke patients with large pretreatment diffusion-weighted imaging lesions may benefit from endovascular treatment. Stroke. 2015; 46:2510–2516.
Article7. Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016; 355:i4919.
Article8. Campbell BC, Majoie CB, Albers GW, Menon BK, Yassi N, Sharma G, et al. Penumbral imaging and functional outcome in patients with anterior circulation ischaemic stroke treated with endovascular thrombectomy versus medical therapy: a meta-analysis of individual patient-level data. Lancet Neurol. 2019; 18:46–55.9. Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015; 372:11–20.10. Bracard S, Ducrocq X, Mas JL, Soudant M, Oppenheim C, Moulin T, et al. Mechanical thrombectomy after intravenous alteplase versus alteplase alone after stroke (THRACE): a randomised controlled trial. Lancet Neurol. 2016; 15:1138–1147.
Article11. Sterne JA, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019; 366:l4898.
Article12. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007; 147:573–577.
Article13. Hacke W, Kaste M, Fieschi C, von Kummer R, Davalos A, Meier D, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australasian Acute Stroke Study Investigators. Lancet. 1998; 352:1245–1251.14. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986; 7:177–188.
Article15. Gautheron V, Xie Y, Tisserand M, Raoult H, Soize S, Naggara O, et al. Outcome after reperfusion therapies in patients with large baseline diffusion-weighted imaging stroke lesions: a THRACE trial (mechanical thrombectomy after intravenous alteplase versus alteplase alone after stroke) subgroup analysis. Stroke. 2018; 49:750–753.
Article16. Rebello LC, Bouslama M, Haussen DC, Dehkharghani S, Grossberg JA, Belagaje S, et al. Endovascular treatment for patients with acute stroke who have a large ischemic core and large mismatch imaging profile. JAMA Neurol. 2017; 74:34–40.
Article17. Haussen DC, Dehkharghani S, Rangaraju S, Rebello LC, Bouslama M, Grossberg JA, et al. Automated CT perfusion ischemic core volume and noncontrast CT ASPECTS (Alberta stroke program early CT score): correlation and clinical outcome prediction in large vessel stroke. Stroke. 2016; 47:2318–2322.18. Panni P, Gory B, Xie Y, Consoli A, Desilles JP, Mazighi M, et al. Acute stroke with large ischemic core treated by thrombectomy. Stroke. 2019; 50:1164–1171.19. Sarraj A, Hassan AE, Savitz S, Sitton C, Grotta J, Chen P, et al. Outcomes of endovascular thrombectomy vs medical management alone in patients with large ischemic cores: a secondary analysis of the optimizing patient's selection for endovascular treatment in acute ischemic stroke (SELECT) study. JAMA Neurol. 2019; 76:1147–1156.20. Yoshimoto T, Inoue M, Tanaka K, Kanemaru K, Koge J, Shiozawa M, et al. Identifying large ischemic core volume ranges in acute stroke that can benefit from mechanical thrombectomy. J Neurointerv Surg. 2020 Dec 15 [Epub]. https://doi.org/10.1136/neurintsurg-2020-016934.
Article21. Chen Z, Zhang R, Zhou Y, Gong X, Zhang M, Shi F, et al. Patients with ischemic core ≥70 ml within 6 h of symptom onset may still benefit from endovascular treatment. Front Neurol. 2018; 9:933.
Article22. Hassen WB, Tisserand M, Turc G, Charron S, Seners P, Edjlali M, et al. Comparison between voxel-based and subtraction methods for measuring diffusion-weighted imaging lesion growth after thrombolysis. Int J Stroke. 2016; 11:221–228.
Article23. Sarraj A, Grotta JC, Pujara DK, Shaker F, Tsivgoulis G. Triage imaging and outcome measures for large core stroke thrombectomy: a systematic review and meta-analysis. J Neurointerv Surg. 2020; 12:1172–1179.24. Demeestere J, Garcia-Esperon C, Garcia-Bermejo P, Ombelet F, McElduff P, Bivard A, et al. Evaluation of hyperacute infarct volume using ASPECTS and brain CT perfusion core volume. Neurology. 2017; 88:2248–2253.
Article25. de Margerie-Mellon C, Turc G, Tisserand M, Naggara O, Calvet D, Legrand L, et al. Can DWI-ASPECTS substitute for lesion volume in acute stroke? Stroke. 2013; 44:3565–3567.
Article26. Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, et al. Endovascular thrombectomy after largevessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016; 387:1723–1731.
Article27. Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018; 378:11–21.28. Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, OrtegaGutierrez S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018; 378:708–718.
Article29. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. 2018 Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018; 49:e46–e110.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Safety and Efficacy of Mechanical Thrombectomy with Solitaire Stent Retrieval for Acute Ischemic Stroke: A Systematic Review
- Mechanical Thrombectomy in Strokes with Large-Vessel Occlusion Beyond 6 Hours: A Pooled Analysis of Randomized Trials
- Mechanical Thrombectomy for In-Hospital Onset Stroke: A Comparative Systematic Review and Meta-Analysis
- Endovascular Thrombectomy for Large Ischemic Strokes: A Living Systematic Review and Meta-Analysis of Randomized Trials
- Paradigm Shift in Intra-Arterial Mechanical Thrombectomy for Acute Ischemic Stroke : A Review of Randomized Controlled Trials after 2015