J Pathol Transl Med.
2021 Sep;55(5):307-316. 10.4132/jptm.2021.05.11.
SMARCA4/BRG1 protein-deficient thoracic tumors dictate re-examination of small biopsy reporting in non–small cell lung cancer
- Affiliations
-
- 1Department of Laboratory, Molecular and Transfusion Services, Rajiv Gandhi Cancer Institute and Research Centre (RGCIRC), New Delhi, India
- 2Department of Pathology, Rajiv Gandhi Cancer Institute and Research Centre (RGCIRC), New Delhi, India
- 3Department of Research, Rajiv Gandhi Cancer Institute and Research Centre (RGCIRC), New Delhi, India
- 4Department of Radiology, Rajiv Gandhi Cancer Institute and Research Centre (RGCIRC), New Delhi, India
- KMID: 2520169
- DOI: http://doi.org/10.4132/jptm.2021.05.11
Abstract
- Background
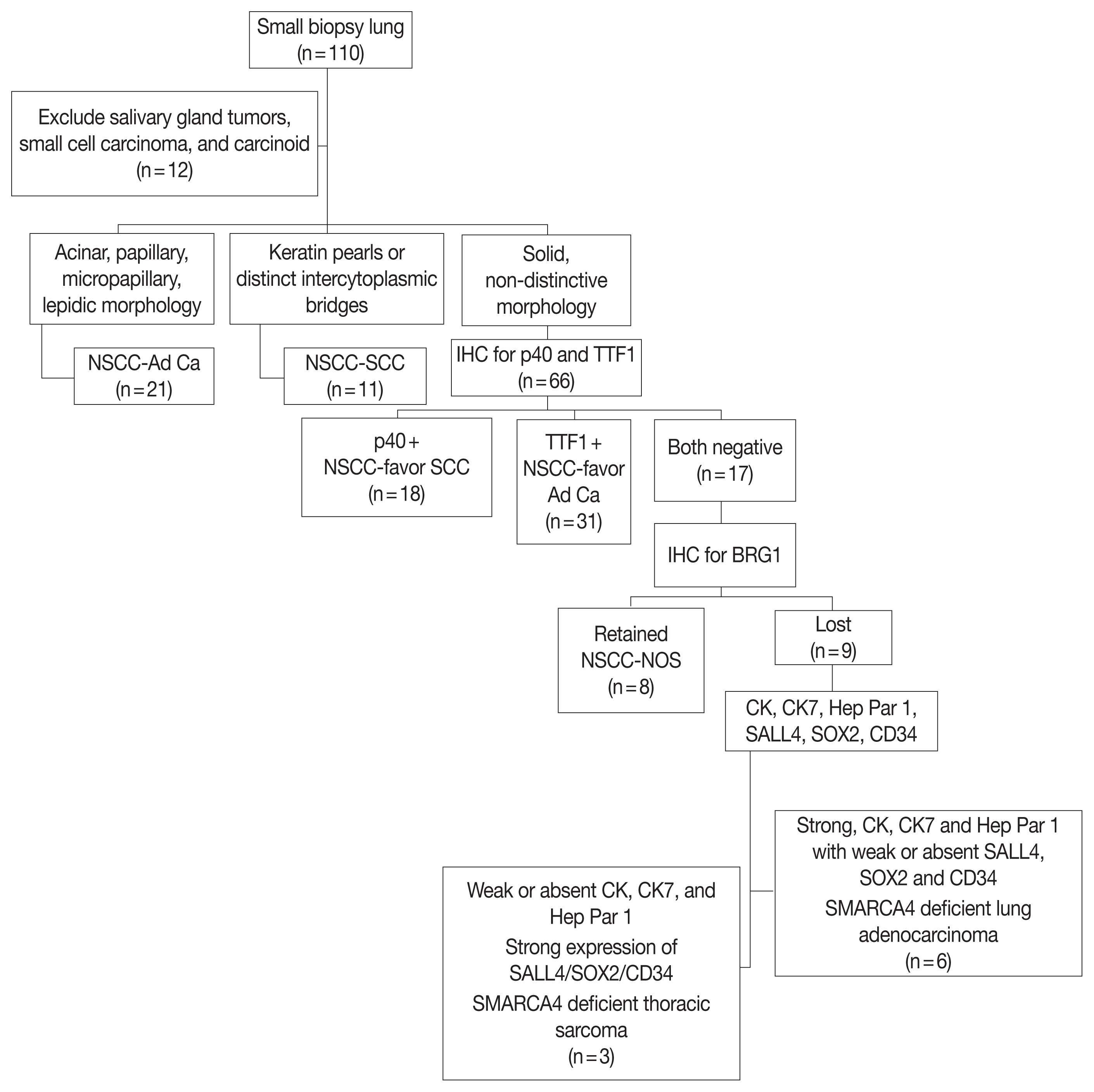
SMARCA4/BRG1 protein–deficient lung adenocarcinomas and thoracic sarcoma are recently described entities that lack distinctive histological features, transcription termination factor 1 (TTF1) reactivity, and actionable driver mutations. The current diagnostic path for small lung biopsies as recommended by the World Health Organization (WHO, 2015) is likely to categorize these as non– small cell carcinoma–not otherwise specified (NSCC-NOS). The present study attempts to define the subtle but distinctive clinicopathologic features of SMARCA4/BRG1 protein-deficient thoracic tumors; highlight their unique biology; and addresses the unmet need to segregate these using a new, tissue-proficient diagnostic pathway.
Methods
All lung biopsies and those from metastatic sites in patients with suspected advanced lung cancer and classified as NSCC-NOS as per WHO (2015) guidelines were subjected to BRG1 testing by immunohistochemistry. SMARCA4/BRG1 protein–deficient thoracic tumors were evaluated by an extended immunohistochemistry panel. Predictive biomarker and programmed death–ligand 1 testing was conducted in all cases.
Results
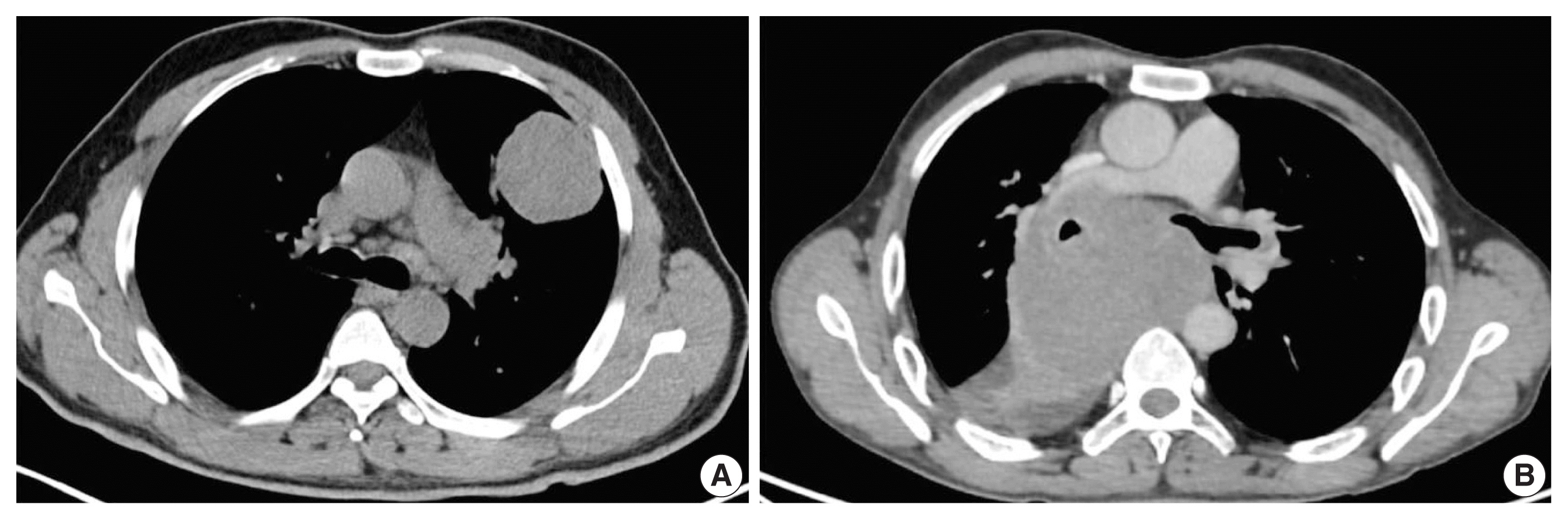
Of 110 cases, nine were found to be SMARCA4/BRG1 protein-deficient; six were identified as SMARCA4/BRG1 protein–deficient lung adenocarcinomas, and three were SMARCA4/BRG1 protein-deficient thoracic sarcomas. The histology ranged from poorly differentiated to undifferentiated to rhabdoid. None of the cases showed significant expression of TTF1 or p40, and no actionable mutation was identified.
Conclusions
It is difficult to separate BRG1-deficient lung adenocarcinomas and thoracic sarcomas based on morphology alone. We propose a diagnostic pathway for small biopsies of thoracic tumors to segregate these distinct entities so that they can be studied more efficaciously for new biomarkers and therapeutic options.
Figure
Cited by 1 articles
-
TTF1-positive
SMARCA4 /BRG1 deficient lung adenocarcinoma
Anurag Mehta, Himanshi Diwan, Divya Bansal, Manoj Gupta
J Pathol Transl Med. 2022;56(1):53-56. doi: 10.4132/jptm.2021.09.16.
Reference
-
References
1. Oike T, Ogiwara H, Nakano T, Yokota J, Kohno T. Inactivating mutations in SWI/SNF chromatin remodeling genes in human cancer. Jpn J Clin Oncol. 2013; 43:849–55.
Article2. Shain AH, Pollack JR. The spectrum of SWI/SNF mutations, ubiquitous in human cancers. PLoS One. 2013; 8:e55119.
Article3. Wang X, Haswell JR, Roberts CW. Molecular pathways: SWI/SNF (BAF) complexes are frequently mutated in cancer: mechanisms and potential therapeutic insights. Clin Cancer Res. 2014; 20:21–7.4. Ganguly D, Sims M, Cai C, Fan M, Pfeffer LM. Chromatin remodeling factor BRG1 regulates stemness and chemosensitivity of glioma initiating cells. Stem Cells. 2018; 36:1804–15.
Article5. Agaimy A. The expanding family of SMARCB1(INI1)-deficient neoplasia: implications of phenotypic, biological, and molecular heterogeneity. Adv Anat Pathol. 2014; 21:394–410.6. Jelinic P, Mueller JJ, Olvera N, et al. Recurrent SMARCA4 mutations in small cell carcinoma of the ovary. Nat Genet. 2014; 46:424–6.
Article7. Stewart CJ, Crook ML. SWI/SNF complex deficiency and mismatch repair protein expression in undifferentiated and dedifferentiated endometrial carcinoma. Pathology. 2015; 47:439–45.
Article8. Agaimy A, Daum O, Markl B, Lichtmannegger I, Michal M, Hartmann A. SWI/SNF Complex-deficient undifferentiated/rhabdoid carcinomas of the gastrointestinal tract: a series of 13 cases highlighting mutually exclusive loss of SMARCA4 and SMARCA2 and frequent co-inactivation of SMARCB1 and SMARCA2. Am J Surg Pathol. 2016; 40:544–53.9. AACR Project GENIE Consortium. AACR Project GENIE: powering precision medicine through an international consortium. Cancer Discov. 2017; 7:818–31.10. Herpel E, Rieker RJ, Dienemann H, et al. SMARCA4 and SMARCA2 deficiency in non-small cell lung cancer: immunohistochemical survey of 316 consecutive specimens. Ann Diagn Pathol. 2017; 26:47–51.
Article11. Agaimy A, Fuchs F, Moskalev EA, Sirbu H, Hartmann A, Haller F. SMARCA4-deficient pulmonary adenocarcinoma: clinicopathological, immunohistochemical, and molecular characteristics of a novel aggressive neoplasm with a consistent TTF1(neg)/CK7(pos)/Hep-Par-1(pos) immunophenotype. Virchows Arch. 2017; 471:599–609.
Article12. Nambirajan A, Singh V, Bhardwaj N, Mittal S, Kumar S, Jain D. SMARCA4/BRG1-deficient non-small cell lung carcinomas: a case series and review of the literature. Arch Pathol Lab Med. 2021; 145:90–8.
Article13. Le Loarer F, Watson S, Pierron G, et al. SMARCA4 inactivation defines a group of undifferentiated thoracic malignancies transcriptionally related to BAF-deficient sarcomas. Nat Genet. 2015; 47:1200–5.
Article14. Perret R, Chalabreysse L, Watson S, et al. SMARCA4-deficient thoracic sarcomas: clinicopathologic study of 30 cses with an emphasis on their nosology and differential diagnoses. Am J Surg Pathol. 2019; 43:455–65.15. Yoshida A, Kobayashi E, Kubo T, et al. Clinicopathological and molecular characterization of SMARCA4-deficient thoracic sarcomas with comparison to potentially related entities. Mod Pathol. 2017; 30:797–809.
Article16. Sauter JL, Graham RP, Larsen BT, Jenkins SM, Roden AC, Boland JM. SMARCA4-deficient thoracic sarcoma: a distinctive clinicopathological entity with undifferentiated rhabdoid morphology and aggressive behavior. Mod Pathol. 2017; 30:1422–32.
Article17. Rekhtman N, Montecalvo J, Chang JC, et al. SMARCA4-deficient thoracic sarcomatoid tumors represent primarily smoking-related undifferentiated carcinomas rather than primary thoracic sarcomas. J Thorac Oncol. 2020; 15:231–47.
Article18. Nicholsan AG, Geisinger K, Aisner SC, et al. Terminology and criteria in non-resection specimens. Travis WD, Brambilla E, Burke AP, Marx A, Nicholson AG, editors. WHO classification of tumors of the lung, pleura, thymus and heart. 4th ed.Lyon: IARC Press;2015. p. 26–37.19. Matsubara D, Kishaba Y, Ishikawa S, et al. Lung cancer with loss of BRG1/BRM, shows epithelial mesenchymal transition phenotype and distinct histologic and genetic features. Cancer Sci. 2013; 104:266–73.
Article20. Mehta A, Saifi M, Batra U, Suryavanshi M, Gupta K. Incidence of ROS1-rearranged non-small-cell lung carcinoma in India and efficacy of crizotinib in lung adenocarcinoma patients. Lung Cancer (Auckl). 2020; 11:19–25.21. Cancer Genome Atlas Research Network. Comprehensive molecular profiling of lung adenocarcinoma. Nature. 2014; 511:543–50.22. Bell EH, Chakraborty AR, Mo X, et al. SMARCA4/BRG1 is a novel prognostic biomarker predictive of cisplatin-based chemotherapy outcomes in resected non-small cell lung cancer. Clin Cancer Res. 2016; 22:2396–404.
Article23. Chan-Penebre E, Armstrong K, Drew A, et al. Selective killing of SMARCA2- and SMARCA4-deficient small cell carcinoma of the ovary, hypercalcemic type cells by inhibition of EZH2: in vitro and in vivo preclinical models. Mol Cancer Ther. 2017; 16:850–60.
Article24. Naito T, Udagawa H, Umemura S, et al. Non-small cell lung cancer with loss of expression of the SWI/SNF complex is associated with aggressive clinicopathological features, PD-L1-positive status, and high tumor mutation burden. Lung Cancer. 2019; 138:35–42.
Article25. Takada K, Sugita S, Murase K, et al. Exceptionally rapid response to pembrolizumab in a SMARCA4-deficient thoracic sarcoma over-expressing PD-L1: a case report. Thorac Cancer. 2019; 10:2312–5.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- TTF1-positive SMARCA4/BRG1 deficient lung adenocarcinoma
- A Case of Spontaneous Regression of Small Cell Lung Cancer
- A Case of Metastatic Gastric Cancer Resulting from Small Cell Lung Cancer
- Fine Needle Aspiration Cytologic Findings of Pulmonary Neuroendocrine Tumors
- Intussusception Induced by Cecal Metastasis of Primary Small Cell Lung Cancer