J Korean Med Sci.
2021 Sep;36(36):e246. 10.3346/jkms.2021.36.e246.
Latent Tuberculosis Infection Screening and Treatment in Congregate Settings (TB FREE COREA): Demographic Profiles of InterferonGamma Release Assay Cohort
- Affiliations
-
- 1Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Incheon St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 2Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Daejeon St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 3Department of Occupational & Environmental Medicine, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 4Department of Urology, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 5Department of Preventive Medicine, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 6Division of Tuberculosis Prevention and Control, Korea Disease Control and Prevention Agency, Cheongju, Korea
- 7Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 8Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Ilsan Paik Hospital, Inje University College of Medicine, Goyang, Korea
- 9Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Dankook University College of Medicine, Cheonan, Korea
- KMID: 2520110
- DOI: http://doi.org/10.3346/jkms.2021.36.e246
Abstract
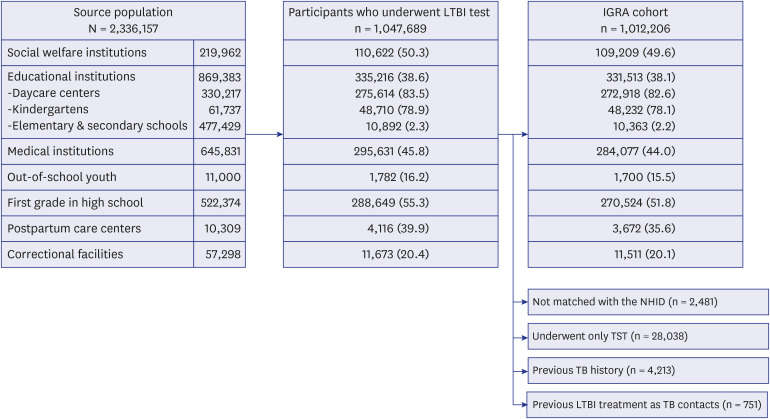
- In 2017, the Korean government launched an unprecedentedly large-scaled latent tuberculosis infection (LTBI) screening project which covered more than a million individuals in congregate settings. A total of 1,047,689 participants of source population (n = 2,336,157) underwent LTBI testing from 2017 to 2018. The overall LTBI test uptake rate during this project was 44.8%. Workers in daycare centers (83.5%) and kindergartens (78.9%) showed high participation rate. A total of 1,012,206 individuals with valid results of interferongamma release assay (IGRA) were selected to constitute the IGRA cohort. Most of the enrolled participants in the IGRA cohort were in their working age. Approximately, threequarters of total enrolled population were female. Investigating the LTBI prevalence, stages of LTBI care cascade, natural history of LTBI, efficacy of LTBI treatment and cost-effectiveness of LTBI screening are feasible within this IGRA cohort.
Figure
Reference
-
1. World Health Organization (WHO). Latent Tuberculosis Infection: Updated and Consolidated Guidelines for Programmatic Management. Geneva, Switzerland: WHO;2018.2. Houben RM, Dodd PJ. The global burden of latent tuberculosis infection: a re-estimation using mathematical modelling. PLoS Med. 2016; 13(10):e1002152. PMID: 27780211.
Article3. Dye C, Glaziou P, Floyd K, Raviglione M. Prospects for tuberculosis elimination. Annu Rev Public Health. 2013; 34(1):271–286. PMID: 23244049.
Article4. GBD Tuberculosis Collaborators. Global, regional, and national burden of tuberculosis, 1990–2016: results from the Global Burden of Diseases, Injuries, and Risk Factors 2016 Study. Lancet Infect Dis. 2018; 18(12):1329–1349. PMID: 30507459.5. Go U, Park M, Kim UN, Lee S, Han S, Lee J, et al. Tuberculosis prevention and care in Korea: evolution of policy and practice. J Clin Tuberc Other Mycobact Dis. 2018; 11:28–36. PMID: 31720389.
Article6. Min J, Kim HW, Stagg HR, Lipman M, Rangaka MX, Myong JP, et al. Latent tuberculosis infection screening and treatment in congregate settings (TB FREE COREA): protocol for a prospective observational study in Korea. BMJ Open. 2020; 10(2):e034098.
Article7. Korean Statistical Information Service. Accessed April 15, 2021. https://kosis.kr/index/index.do.8. Ministry of Health and Welfare, Republic of Korea. Health and Welfare Statistical Yearbook 2018. Sejong, Korea: Ministry of Health and Welfare;2018.9. Ministry of Health and Welfare, Republic of Korea. National Prevention and Control Policy for Tuberculosis among the Staffs of Postpartum Care Centers Is Implemented. Sejong, Korea: Ministry of Health and Welfare;2015.10. Ministry of Justice, Republic of Korea. Korea Correctional Service Statistics 2018. Gwacheon, Korea: Ministry of Justice;2018.11. Seong SC, Kim YY, Khang YH, Park JH, Kang HJ, Lee H, et al. Data resource profile: the National Health Information Database of the National Health Insurance Service in South Korea. Int J Epidemiol. 2017; 46(3):799–800. PMID: 27794523.12. Song JH, Huh K, Chung DR. Modern history of tuberculosis in Korea. Infect Chemother. 2019; 51(4):414–426. PMID: 31782276.
Article13. Kang YA, Lee HW, Yoon HI, Cho B, Han SK, Shim YS, et al. Discrepancy between the tuberculin skin test and the whole-blood interferon γ assay for the diagnosis of latent tuberculosis infection in an intermediate tuberculosis-burden country. JAMA. 2005; 293(22):2756–2761. PMID: 15941805.
Article14. Edwards PQ. Tuberculin testing of children. Pediatrics. 1974; 54(5):628–630. PMID: 4453466.
Article15. Jenkins D, Davidson FF. Isoniazid chemoprophylaxis of tuberculosis. Calif Med. 1972; 116(4):1–5.16. Starke JR. Universal screening for tuberculosis infection. School's out! JAMA. 1995; 274(8):652–653. PMID: 7637147.
Article17. Screening for tuberculosis and tuberculosis infection in high-risk populations. Recommendations of the Advisory Council for the Elimination of Tuberculosis. MMWR Recomm Rep. 1995; 44(RR-11):19–34. PMID: 7565540.18. Targeted tuberculin testing and treatment of latent tuberculosis infection. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. This is a Joint Statement of the American Thoracic Society (ATS) and the Centers for Disease Control and Prevention (CDC). This statement was endorsed by the Council of the Infectious Diseases Society of America. (IDSA), September 1999, and the sections of this statement. Am J Respir Crit Care Med. 2000; 161(4 Pt 2):S221–S247. PMID: 10764341.19. Mori T. Reform of Japan's NTP and its technical perspectives. Kekkaku. 2004; 79(10):587–604. PMID: 15631111.20. Nakatani H, Fujii N, Mori T, Hoshinot H. Epidemiological transition of tuberculosis and future agenda of control in Japan: results of the Ad-Hoc National Survey of Tuberculosis 2000. Int J Tuberc Lung Dis. 2002; 6(3):198–207. PMID: 11934137.21. Ushio M. Amendment of tuberculosis prevention law and prospect of tuberculosis control program. Kekkaku. 2005; 80(7):541–546. PMID: 16167781.22. Korean Educational Development Institute. Data Collection for Education Statistics, 2017. Jincheon, Korea: Korean Educational Development Institute;2017.23. Lönnroth K, Migliori GB, Abubakar I, D'Ambrosio L, de Vries G, Diel R, et al. Towards tuberculosis elimination: an action framework for low-incidence countries. Eur Respir J. 2015; 45(4):928–952. PMID: 25792630.24. Ronald LA, Campbell JR, Rose C, Balshaw R, Romanowski K, Roth DZ, et al. Estimated impact of World Health Organization latent tuberculosis screening guidelines in a region with a low tuberculosis incidence: retrospective cohort study. Clin Infect Dis. 2019; 69(12):2101–2108. PMID: 30856258.
Article25. Campbell JR, Dowdy D, Schwartzman K. Treatment of latent infection to achieve tuberculosis elimination in low-incidence countries. PLoS Med. 2019; 16(6):e1002824. PMID: 31170161.
Article26. European Centre for Disease Prevention and Control (ECDC). Programmatic Management of Latent Tuberculosis Infection in the European Union. Stockholm, Sweden: ECDC;2018.27. National Institute for Health and Care Excellence. Tuberculosis, NICE guideline [NG33]. Accessed April 15, 2021. https://www.nice.org.uk/guidance/ng33.28. Public Health Agency of Canada. Canadian Tuberculosis Standards. 7th Edition. Ottawa, Canada: Government of Canada;2014.29. US Preventive Services Task Force. Bibbins-Domingo K, Grossman DC, Curry SJ, Bauman L, Davidson KW, et al. Screening for latent tuberculosis infection in adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2016; 316(9):962–969. PMID: 27599331.30. Park S. Prioritizing the Target Population for Screening of Latent Tuberculosis Infection through ICER (Incremental Cost Effectiveness Ratio) in Korea. Seoul, Korea: Yonsei University;2016.31. Korean National Tuberculosis Association. 7th Korea National Health and Nutrition Examination Survey, 1st Year (2016): Tuberculin Survey Support and Quality Control. Seoul, Korea: Korea Centers for Disease Control and Prevention;2017.32. Alsdurf H, Hill PC, Matteelli A, Getahun H, Menzies D. The cascade of care in diagnosis and treatment of latent tuberculosis infection: a systematic review and meta-analysis. Lancet Infect Dis. 2016; 16(11):1269–1278. PMID: 27522233.
Article33. Andrews JR, Nemes E, Tameris M, Landry BS, Mahomed H, McClain JB, et al. Serial QuantiFERON testing and tuberculosis disease risk among young children: an observational cohort study. Lancet Respir Med. 2017; 5(4):282–290. PMID: 28215501.
Article34. Gupta RK, Lipman M, Jackson C, Sitch AJ, Southern J, Drobniewski F, et al. Quantitative IFN-γ release assay and tuberculin skin test results to predict incident tuberculosis. a prospective cohort study. Am J Respir Crit Care Med. 2020; 201(8):984–991. PMID: 31825645.
Article35. Winje BA, White R, Syre H, Skutlaberg DH, Oftung F, Mengshoel AT, et al. Stratification by interferon-γ release assay level predicts risk of incident TB. Thorax. 2018; 73(7):652–661.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Diagnosis and Treatment of Latent Tuberculosis Infection in Healthcare Workers
- Diagnosis and treatment of latent tuberculosis infection
- Good Agreement between an Interferon Gamma Release Assay and Tuberculin Skin Tests in Testing for Latent Tuberculosis Infection among HIV-Infected Patients in Indonesia
- Preventing the Transmission of Tuberculosis in Health Care Settings: Administrative Control
- Diagnosis and Treatment of Latent Tuberculosis Infection