J Korean Neurosurg Soc.
2021 Sep;64(5):716-725. 10.3340/jkns.2021.0068.
Propranolol Inhibits the Proliferation of Human Glioblastoma Cell Lines through Notch1 and Hes1 Signaling System
- Affiliations
-
- 1Department of Neurosurgery, Hallym University Sacred Heart Hospital, Anyang, Korea
- 2Department of Obstetrics and Gynecology, Hallym University Sacred Heart Hospital, Anyang, Korea
- 3Department of Pathology, Hallym University Sacred Heart Hospital, Anyang, Korea
- KMID: 2519698
- DOI: http://doi.org/10.3340/jkns.2021.0068
Abstract
Objective
: The anti-tumor effect of the beta-adrenergic receptor antagonist propranolol in breast cancer is well known; however, its activity in glioblastoma is not well-evaluated. The Notch-Hes pathway is known to regulate cell differentiation, proliferation, and apoptosis. We investigated the effect of propranolol to human glioblastoma cell lines, and the role of Notch and Hes signaling in this process.
Methods
: We performed immunohistochemical staining on 31 surgically resected primary human glioblastoma tissues. We also used glioblastoma cell lines of U87-MG, LN229, and neuroblastoma cell line of SH-SY5Y in this study. The effect of propranolol and isoproterenol on cell proliferation was evaluated using the MTT assay (absorbance 570 nm). The impact of propranolol on gene expression (Notch and Hes) was evaluated using real-time polymerase chain reaction (RT-PCR, whereas protein levels of Notch1 and Hes1 were measured using Western blotting (WB), simultaneously. Small interfering RNA (siRNA) was used to suppress the Notch gene to investigate its role in the proliferation of glioblastoma.
Results
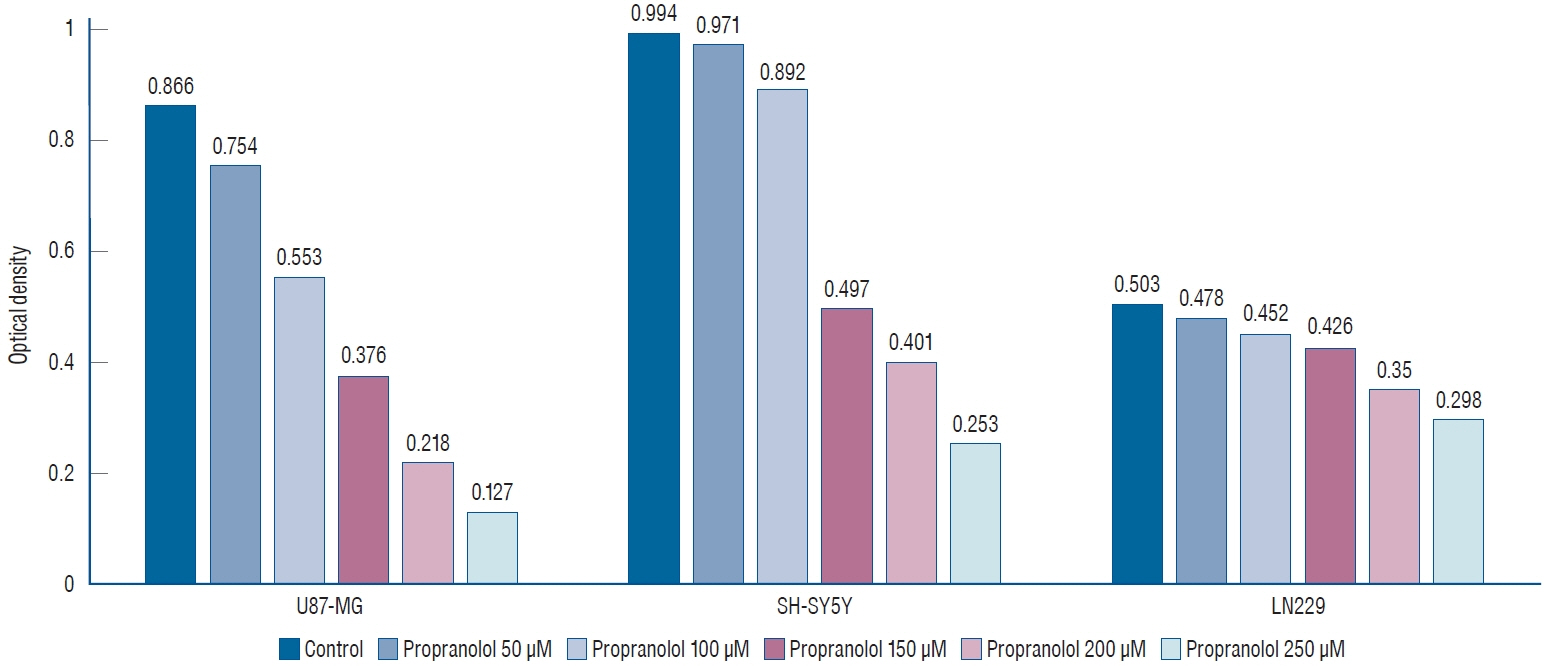
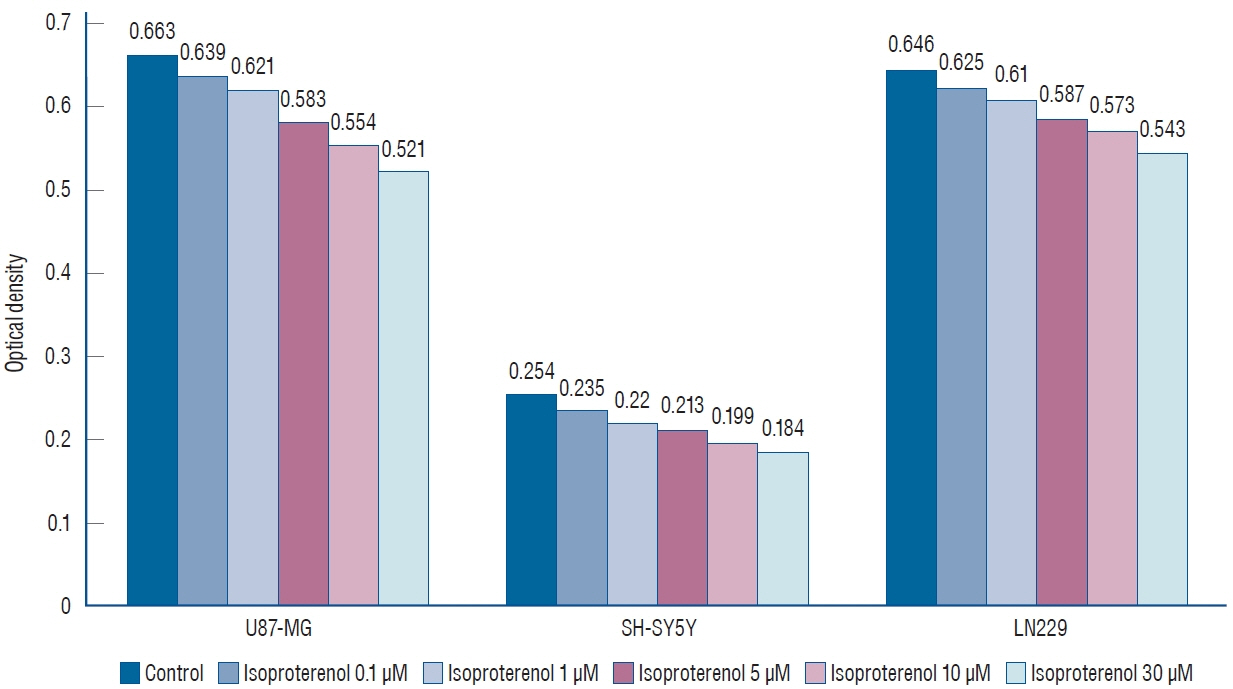
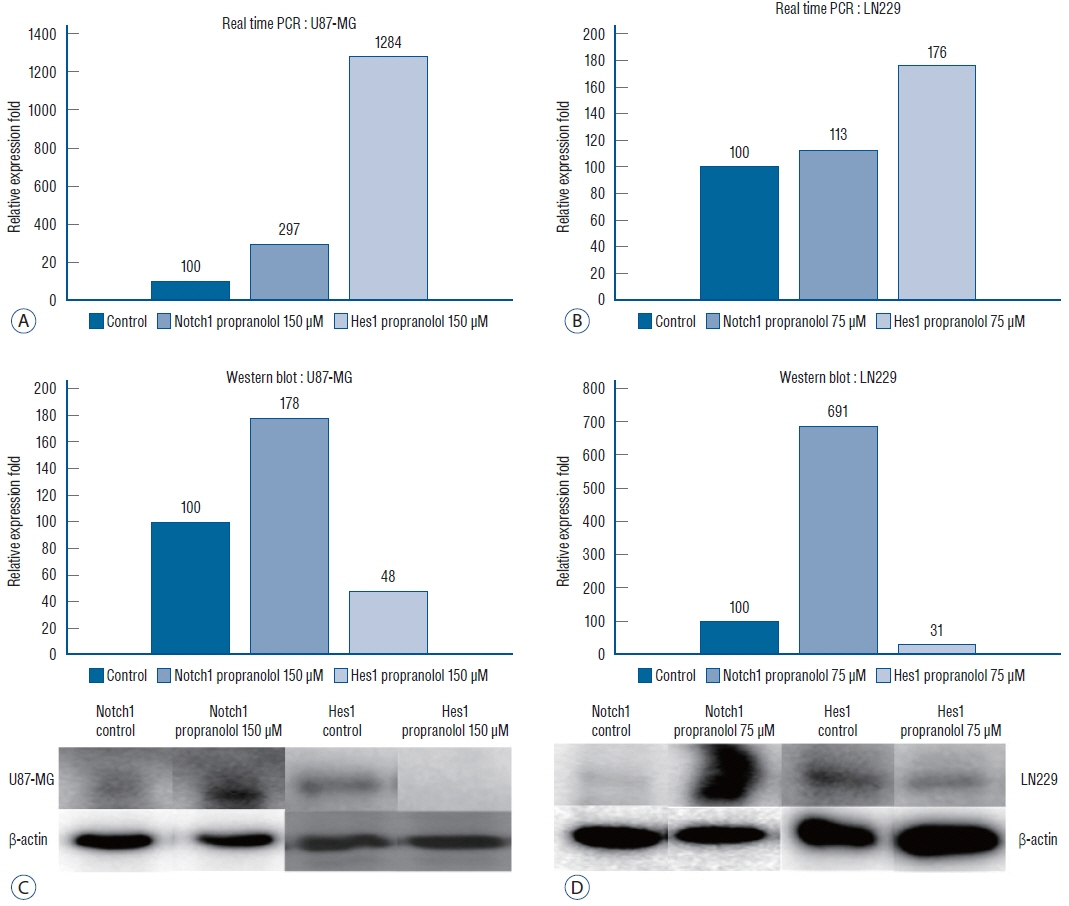
: Propranolol and isoproterenol caused a dose-dependent decrease in cell proliferation (MTT assay). RT-PCR showed an increase in Notch1 and Hes1 expression by propranolol, whereas WB demonstrated increase in Notch1 protein, but a decrease in Hes1 by propranolol. The proliferation of U87-MG and LN229 was not significantly suppressed after transfection with Notch siRNA.
Conclusion
: These results demonstrated that propranolol suppressed the proliferation of glioblastoma cell lines and neuroblastoma cell line, and Hes1 was more closely involved than Notch1 was in glioblastoma proliferation.
Keyword
Figure
Reference
-
References
1. Algazi M, Plu-Bureau G, Flahault A, Dondon MG, Lê MG. Could treatments with beta-blockers be associated with a reduction in cancer risk? Rev Epidemiol Sante Publique. 52:53–65. 2004.2. Anjum K, Shagufta BI, Abbas SQ, Patel S, Khan I, Shah SAA, et al. Current status and future therapeutic perspectives of glioblastoma multiforme (GBM) therapy: a review. Biomed Pharmacother. 92:681–689. 2017.
Article3. Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 284:770–776. 1999.
Article4. Aster JC, Pear WS. Notch signaling in leukemia. Curr Opin Hematol. 8:237–244. 2001.
Article5. Bazzoni R, Bentivegna A. Role of notch signaling pathway in glioblastoma pathogenesis. Cancers (Basel). 11:292. 2019.6. Bigas A, Martin DI, Milner LA. Notch1 and Notch2 inhibit myeloid differentiation in response to different cytokines. Mol Cell Biol. 18:2324–2333. 1998.
Article7. Bolós V, Grego-Bessa J, de la Pompa JL. Notch signaling in development and cancer. Endocr Rev. 28:339–363. 2007.
Article8. Cheung HC, Corley LJ, Fuller GN, McCutcheon IE, Cote GJ. Polypyrimidine tract binding protein and Notch1 are independently re-expressed in glioma. Mod Pathol. 19:1034–1041. 2006.
Article9. Dell’albani P, Rodolico M, Pellitteri R, Tricarichi E, Torrisi SA, D’Antoni S, et al. Differential patterns of NOTCH1-4 receptor expression are markers of glioma cell differentiation. Neuro Oncol. 16:204–216. 2014.
Article10. Fassberg J, Stella VJ. A kinetic and mechanistic study of the hydrolysis of camptothecin and some analogues. J Pharm Sci. 81:676–684. 1992.
Article11. Hai L, Zhang C, Li T, Zhou X, Liu B, Li S, et al. Notch1 is a prognostic factor that is distinctly activated in the classical and proneural subtype of glioblastoma and that promotes glioma cell survival via the NFκB(p65) pathway. Cell Death Dis. 9:158. 2018.
Article12. Harada K, Sato Y, Ikeda H, Hsu M, Igarashi S, Nakanuma Y. Notch1- Hes1 signalling axis in the tumourigenesis of biliary neuroendocrine tumours. J Clin Pathol. 66:386–391. 2013.
Article13. He JJ, Zhang WH, Liu SL, Chen YF, Liao CX, Shen QQ, et al. Activation of β-adrenergic receptor promotes cellular proliferation in human glioblastoma. Oncol Lett. 14:3846–3852. 2017.
Article14. Hiller JG, Cole SW, Crone EM, Byrne DJ, Shackleford DM, Pang JB, et al. Preoperative β-blockade with propranolol reduces biomarkers of metastasis in breast cancer: a phase II randomized trial. Clin Cancer Res. 26:1803–1811. 2020.
Article15. Jang MS, Zlobin A, Kast WM, Miele L. Notch signaling as a target in multimodality cancer therapy. Curr Opin Mol Ther. 2:55–65. 2000.16. Jundt F, Anagnostopoulos I, Förster R, Mathas S, Stein H, Dörken B. Activated Notch1 signaling promotes tumor cell proliferation and survival in Hodgkin and anaplastic large cell lymphoma. Blood. 99:3398–3433. 2002.
Article17. Kim YY, Park CK, Kim SK, Phi JH, Kim JH, Kim CY, et al. CKD-602, a camptothecin derivative, inhibits proliferation and induces apoptosis in glioma cell lines. Oncol Rep. 21:1419–1419. 2009.
Article18. Kopan R, Ilagan MX. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 137:216–233. 2009.
Article19. Krex D, Klink B, Hartmann C, von Deimling A, Pietsch T, Simon M, et al. Long-term survival with glioblastoma multiforme. Brain. 130(Pt 10):2596–2606. 2007.
Article20. Kusaczuk M, Krętowski R, Bartoszewicz M, Cechowska-Pasko M. Phenylbutyrate-a pan-HDAC inhibitor-suppresses proliferation of glioblastoma LN-229 cell line. Tumour Biol. 37:931–942. 2016.
Article21. Leong KG, Karsan A. Recent insights into the role of Notch signaling in tumorigenesis. Blood. 107:2223–2233. 2006.
Article22. Li J, Cui Y, Gao G, Zhao Z, Zhang H, Wang X. Notch1 is an independent prognostic factor for patients with glioma. J Surg Oncol. 103:813–817. 2011.
Article23. Lino MM, Merlo A, Boulay JL. Notch signaling in glioblastoma: a developmental drug target? BMC Med. 8:72. 2010.
Article24. Liu Y, Su C, Shan Y, Yang S, Ma G. Targeting Notch1 inhibits invasion and angiogenesis of human breast cancer cells via inhibition nuclear factor-κB signaling. Am J Transl Res. 8:2681–3692. 2016.25. Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Fuller C. World Health Organization classification of tumours of the central nervous system, fourth edition. J Neuropathol Exp Neurol. 67:260. 2008.
Article26. Louis DN, Perry A, Burger P, Ellison DW, Reifenberger G, von Deimling A, et al. International Society of Neuropathology--haarlem consensus guidelines for nervous system tumor classification and grading. Brain Pathol. 24:429–435. 2014.
Article27. Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 131:803–820. 2016.
Article28. Margareto J, Leis O, Larrarte E, Idoate MA, Carrasco A, Lafuente JV. Gene expression profiling of human gliomas reveals differences between GBM and LGA related to energy metabolism and notch signaling pathways. J Mol Neurosci. 32:53–63. 2007.
Article29. Miele L, Golde T, Osborne B. Notch signaling in cancer. Curr Mol Med. 6:905–918. 2006.
Article30. Nicolas M, Wolfer A, Raj K, Kummer JA, Mill P, van Noort M, et al. Notch1 functions as a tumor suppressor in mouse skin. Nat Genet. 33:416–421. 2003.
Article31. Ostrom QT, Gittleman H, Truitt G, Boscia A, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the united states in 2011-2015. Neuro Oncol. 20(suppl_4):iv1–iv86. 2018.
Article32. Park JC, Chang IB, Ahn JH, Kim JH, Song JH, Moon SM, et al. Nerve growth factor stimulates glioblastoma proliferation through Notch1 receptor signaling. J Korean Neurosurg Soc. 61:441–449. 2018.
Article33. Park YH, Kim SJ, Jeong BH, Herzog TJ, Wright J, Kitajewski J, et al. Follicular stimulating hormone enhances Notch 1 expression in SK-OV-3 ovarian cancer cells. J Gynecol Oncol. 21:119–124. 2010.
Article34. Pasquier E, Ciccolini J, Carre M, Giacometti S, Fanciullino R, Pouchy C, et al. Propranolol potentiates the anti-angiogenic effects and anti-tumor efficacy of chemotherapy agents: implication in breast cancer treatment. Oncotarget. 2:797–809. 2011.
Article35. Pasquier E, Street J, Pouchy C, Carre M, Gifford AJ, Murray J, et al. β-blockers increase response to chemotherapy via direct antitumour and anti-angiogenic mechanisms in neuroblastoma. Br J Cancer. 108:2485–2494. 2013.
Article36. Phillips HS, Kharbanda S, Chen R, Forrest WF, Soriano RH, Wu TD, et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 9:157–173. 2006.
Article37. Powe DG, Voss MJ, Zänker KS, Habashy HO, Green AR, Ellis IO, et al. Beta-blocker drug therapy reduces secondary cancer formation in breast cancer and improves cancer specific survival. Oncotarget. 1:628–638. 2010.
Article38. Rundle-Thiele D, Head R, Cosgrove L, Martin JH. Repurposing some older drugs that cross the blood-brain barrier and have potential anticancer activity to provide new treatment options for glioblastoma. Br J Clin Pharmacol. 81:199–209. 2016.
Article39. Sriuranpong V, Borges MW, Ravi RK, Arnold DR, Nelkin BD, Baylin SB, et al. Notch signaling induces cell cycle arrest in small cell lung cancer cells. Cancer Res. 61:3200–3205. 2001.40. Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 352:987–996. 2005.
Article41. Xu P, Yu S, Jiang R, Kang C, Wang G, Jiang H, et al. Differential expression of Notch family members in astrocytomas and medulloblastomas. Pathol Oncol Res. 15:703–710. 2009.
Article42. Zhang D, Ma Q, Shen S, Hu H. Inhibition of pancreatic cancer cell proliferation by propranolol occurs through apoptosis induction: the study of beta-adrenoceptor antagonist’s anticancer effect in pancreatic cancer cell. Pancreas. 38:94–100. 2009.
Article43. Zhang H, Wei T, Johnson A, Sun R, Richter G, Strub GM. NOTCH pathway activation in infantile hemangiomas. J Vasc Surg Venous Lymphat Disord. 9:489–496. 2021.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Nerve Growth Factor Stimulates Glioblastoma Proliferation through Notch1 Receptor Signaling
- Bacoside A Induced Sub-G0 Arrest and Early Apoptosis in Human Glioblastoma Cell Line U-87 MG through Notch Signaling Pathway
- Interaction of Wnt5a with Notch1 is Critical for the Pathogenesis of Psoriasis
- The Role of Notch1 Signaling in Anaplastic Thyroid Carcinoma
- Effect of Adrenomedullin on Proliferation and Mitogen-Activated Protein Kinase Activity in Osteosarcoma Cell Lines (MG63, U2OS)