J Korean Neurosurg Soc.
2021 Sep;64(5):705-715. 10.3340/jkns.2021.0003.
Optimal Ratio of Wnt3a Expression in Human Mesenchymal Stem Cells Promotes Axonal Regeneration in Spinal Cord Injured Rat Model
- Affiliations
-
- 1Department of Neurological Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 2Department of Biochemistry and Molecular Biology, University of Ulsan College of Medicine, Seoul, Korea
- 3Department of Microbiology, University of Ulsan College of Medicine, Seoul, Korea
- 4Bio-Medical Institute of Technology, University of Ulsan College of Medicine, Seoul, Korea
- KMID: 2519697
- DOI: http://doi.org/10.3340/jkns.2021.0003
Abstract
Objective
: Through our previous clinical trials, the demonstrated therapeutic effects of MSC in chronic spinal cord injury (SCI) were found to be not sufficient. Therefore, the need to develop stem cell agent with enhanced efficacy is increased. We transplanted enhanced Wnt3asecreting human mesenchymal stem cells (hMSC) into injured spines at 6 weeks after SCI to improve axonal regeneration in a rat model of chronic SCI. We hypothesized that enhanced Wnt3a protein expression could augment neuro-regeneration after SCI.
Methods
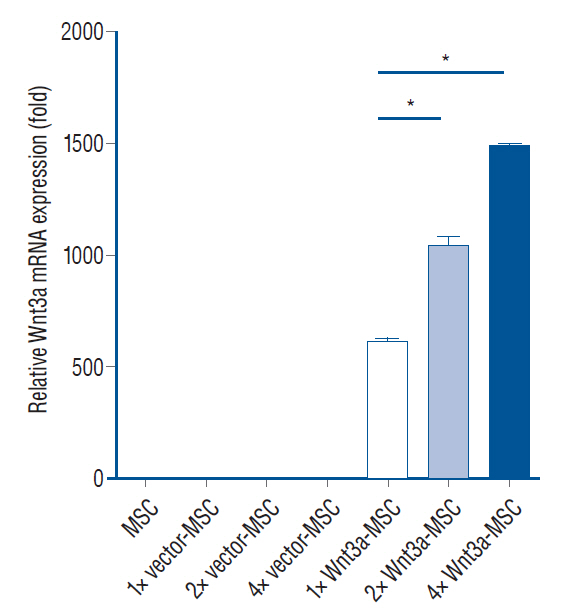
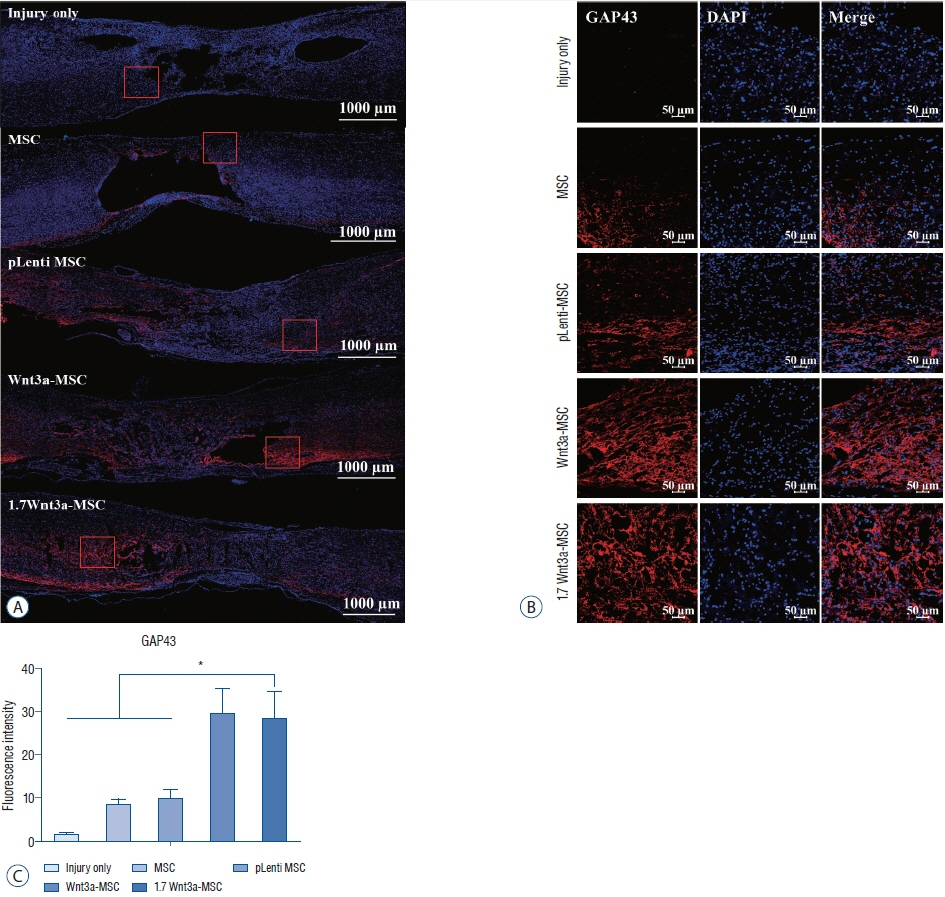
: Thirty-six Sprague-Dawley rats were injured using an Infinite Horizon (IH) impactor at the T9–10 vertebrae and separated into five groups : 1) phosphate-buffered saline injection (injury only group, n=7); 2) hMSC transplantation (MSC, n=7); 3) hMSC transfected with pLenti vector (without Wnt3a gene) transplantation (pLenti-MSC, n=7); 4) hMSC transfected with Wnt3a gene transplantation (Wnt3a-MSC, n=7); and 5) hMSC transfected with enhanced Wnt3a gene (1.7 fold Wnt3a mRNA expression) transplantation (1.7 Wnt3a-MSC, n=8). Six weeks after SCI, each 5×105 cells/15 µL at 2 points were injected using stereotactic and microsyringe pump. To evaluate functional recovery from SCI, rats underwent Basso-Beattie-Bresnahan (BBB) locomotor test on the first, second, and third days post-injury and then weekly for 14 weeks. Axonal regeneration was assessed using growth-associated protein 43 (GAP43), microtubule-associated protein 2 (MAP2), and neurofilament (NF) immunostaining.
Results
: Fourteen weeks after injury (8 weeks after transplantation), BBB score of the 1.7 Wnt3a-MSC group (15.0±0.28) was significantly higher than that of the injury only (10.0±0.48), MSC (12.57±0.48), pLenti-MSC (12.42±0.48), and Wnt3a-MSC (13.71±0.61) groups (p<0.05). Immunostaining revealed increased expression of axonal regeneration markers GAP43, MAP2, and NF in the Wnt3a-MSC and 1.7 Wnt3a-MSC groups.
Conclusion
: Our results showed that enhanced gene expression of Wnt3a in hMSC can potentiate axonal regeneration and improve functional recovery in a rat model of chronic SCI.
Keyword
Figure
Reference
-
References
1. Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 12:1–21. 1995.
Article2. Blits B, Kitay BM, Farahvar A, Caperton CV, Dietrich WD, Bunge MB. Lentiviral vector-mediated transduction of neural progenitor cells before implantation into injured spinal cord and brain to detect their migration, deliver neurotrophic factors and repair tissue. Restor Neurol Neurosci. 23:313–324. 2005.3. Boyce VS, Mendell LM. Neurotrophic factors in spinal cord injury. Handb Exp Pharmacol. 220:443–460. 2014.
Article4. Chao MV. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci. 4:299–309. 2003.
Article5. Dinsmore J, Ratliff J, Deacon T, Pakzaban P, Jacoby D, Galpern W, et al. Embryonic stem cells differentiated in vitro as a novel source of cells for transplantation. Cell Transplant. 5:131–143. 1996.
Article6. Fawcett JW, Curt A, Steeves JD, Coleman WP, Tuszynski MH, Lammertse D, et al. Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: spontaneous recovery after spinal cord injury and statistical power needed for therapeutic clinical trials. Spinal Cord. 45:190–205. 2007.
Article7. Hirabayashi Y, Itoh Y, Tabata H, Nakajima K, Akiyama T, Masuyama N, et al. The Wnt/beta-catenin pathway directs neuronal differentiation of cortical neural precursor cells. Development. 131:2791–2801. 2004.
Article8. Huelsken J, Behrens J. The Wnt signalling pathway. J Cell Sci. 115:3977–3978. 2002.
Article9. Jeong SK, Choi I, Jeon SR. Current status and future strategies to treat spinal cord injury with adult stem cells. J Korean Neurosurg Soc. 63:153–162. 2020.
Article10. Kutikov AB, Moore SW, Layer RT, Podell PE, Sridhar N, Santamaria AJ, et al. Method and apparatus for the automated delivery of continuous neural stem cell trails into the spinal cord of small and large animals. Neurosurgery. 85:560–573. 2019.
Article11. McDonald JW, Liu XZ, Qu Y, Liu S, Mickey SK, Turetsky D, et al. Transplanted embryonic stem cells survive, differentiate and promote recovery in injured rat spinal cord. Nat Med. 5:1410–1412. 1999.
Article12. Mehra A, Lee KH, Hatzimanikatis V. Insights into the relation between mRNA and protein expression patterns: I. Theoretical considerations. Biotechnol Bioeng. 84:822–833. 2003.
Article13. Miyashita T, Koda M, Kitajo K, Yamazaki M, Takahashi K, Kikuchi A, et al. Wnt-Ryk signaling mediates axon growth inhibition and limits functional recovery after spinal cord injury. J Neurotrauma. 26:955–964. 2009.
Article14. Mukhamedshina YO, Akhmetzyanova ER, Kostennikov AA, Zakirova EY, Galieva LR, Garanina EE, et al. Adipose-derived mesenchymal stem cell application combined with fibrin matrix promotes structural and functional recovery following spinal cord injury in rats. Front Pharmacol. 9:343. 2018.
Article15. Nakano N, Nakai Y, Seo TB, Homma T, Yamada Y, Ohta M, et al. Effects of bone marrow stromal cell transplantation through CSF on the subacute and chronic spinal cord injury in rats. PLoS One. 8:e73494. 2013.
Article16. Oh SK, Choi KH, Yoo JY, Kim DY, Kim SJ, Jeon SR. A phase III clinical trial showing limited efficacy of autologous mesenchymal stem cell therapy for spinal cord injury. Neurosurgery. 78:436–447. discussion 447. 2016.
Article17. Ohta M, Suzuki Y, Noda T, Ejiri Y, Dezawa M, Kataoka K, et al. Bone marrow stromal cells infused into the cerebrospinal fluid promote functional recovery of the injured rat spinal cord with reduced cavity formation. Exp Neurol. 187:266–278. 2004.
Article18. Onishi K, Hollis E, Zou Y. Axon guidance and injury-lessons from Wnts and Wnt signaling. Curr Opin Neurobiol. 232–240. 2014.19. Osaka M, Honmou O, Murakami T, Nonaka T, Houkin K, Hamada H, et al. Intravenous administration of mesenchymal stem cells derived from bone marrow after contusive spinal cord injury improves functional outcome. Brain Res. 1343:226–235. 2010.
Article20. Park JH, Kim DY, Sung IY, Choi GH, Jeon MH, Kim KK, et al. Long-term results of spinal cord injury therapy using mesenchymal stem cells derived from bone marrow in humans. Neurosurgery. 70:1238–1247. discussion 1247. 2012.
Article21. Park JH, Min J, Baek SR, Kim SW, Kwon IK, Jeon SR. Enhanced neuroregenerative effects by scaffold for the treatment of a rat spinal cord injury with Wnt3a-secreting fibroblasts. Acta Neurochir (Wien). 155:809–816. 2013.
Article22. Parr BA, Shea MJ, Vassileva G, McMahon AP. Mouse Wnt genes exhibit discrete domains of expression in the early embryonic CNS and limb buds. Development. 119:247–261. 1993.
Article23. Patapoutian A, Reichardt LF. Roles of Wnt proteins in neural development and maintenance. Curr Opin Neurobiol. 10:392–399. 2000.
Article24. Samdani AF, Paul C, Betz RR, Fischer I, Neuhuber B. Transplantation of human marrow stromal cells and mono-nuclear bone marrow cells into the injured spinal cord: a comparative study. Spine (Phila Pa 1976). 34:2605–2612. 2009.
Article25. Seo DK, Kim JH, Min J, Yoon HH, Shin ES, Kim SW, et al. Enhanced axonal regeneration by transplanted Wnt3a-secreting human mesenchymal stem cells in a rat model of spinal cord injury. Acta Neurochir (Wien). 159:947–957. 2017.
Article26. Suh HI, Min J, Choi KH, Kim SW, Kim KS, Jeon SR. Axonal regeneration effects of Wnt3a-secreting fibroblast transplantation in spinal cordinjured rats. Acta Neurochir (Wien). 153:1003–1010. 2011.
Article27. Verma R, Virdi JK, Singh N, Jaggi AS. Animals models of spinal cord contusion injury. Korean J Pain. 32:12–21. 2019.
Article28. Zou Y. Wnt signaling in axon guidance. Trends Neurosci. 27:528–532. 2004.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Olig2-expressing Mesenchymal Stem Cells Enhance Functional Recovery after Contusive Spinal Cord Injury
- Aucubin Promotes Neurite Outgrowth in Neural Stem Cells and Axonal Regeneration in Sciatic Nerves
- Current Concept of Stem Cell Therapy for Spinal Cord Injury: A Review
- Upregulation of C-Reactive Protein by Placenta-Derived Mesenchymal Stem Cells Promotes Angiogenesis in A Rat Model with Cirrhotic Liver
- Comparison of the Outcomes after Intralesional, Intracisternal, and Intravenous Transplantation of Human Bone Marrow Derived Mesenchymal Stem Cells for Spinal Cord Injured Rat