Int J Stem Cells.
2021 Aug;14(3):275-285. 10.15283/ijsc20157.
Bie Jia Jian Pill Combined with Bone Mesenchymal Stem Cells Regulates microRNA-140 to Suppress Hepatocellular Carcinoma Stem Cells
- Affiliations
-
- 1Department of Spleen, Stomach and Liver Diseases, The First Affiliated Hospital of Guangxi University of Chinese Medicine, Nanning, China
- 2Guangxi Key Laboratory of Translational Medicine for Treating High-Incidence Infectious Diseases with Integrative Medicine, Nanning, China
- 3Teaching and Research Office of Internal Medicine of Traditional Chinese Medicine, The First Affiliated Hospital of Guangxi University of Chinese Medicine, Nanning, China
- 4Department of Internal Medicine, The First Affiliated Hospital of Guangxi University of Chinese Medicine, Nanning, China
- 5Department of Human Resources, The First Affiliated Hospital of Guangxi University of Chinese Medicine, Nanning, China
- 6Guangxi Key Laboratory of Basic Research of Traditional Chinese Medicine, Nanning, China
- KMID: 2519370
- DOI: http://doi.org/10.15283/ijsc20157
Abstract
- Background and Objectives
Cancer stem cells (CSCs) with tumorigenic potential are reported as the crucial factors of hepatocellular carcinoma (HCC) recurrence and therapy resistance. Bone mesenchymal stem cells (BMSCs) are documented to play an important role in the protection of hepatocytes. Bie Jia Jian pill (BJJP), a Traditional Chinese Medicine, has been used to treat liver fibrosis and liver cancer. This study aimed to explore the potential role of combined use of BJJP with BMSCs in HCC cell lines.
Methods and Results
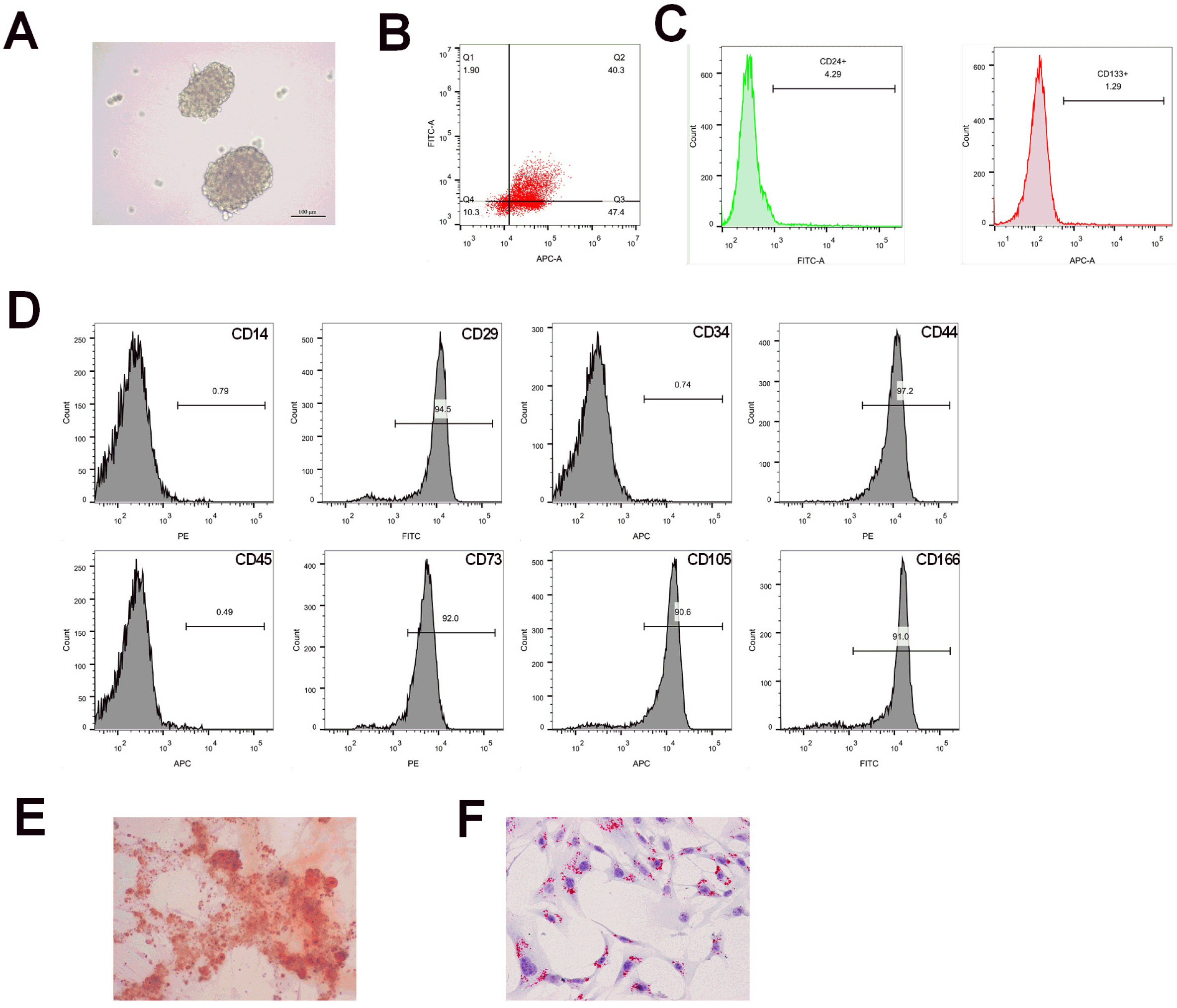
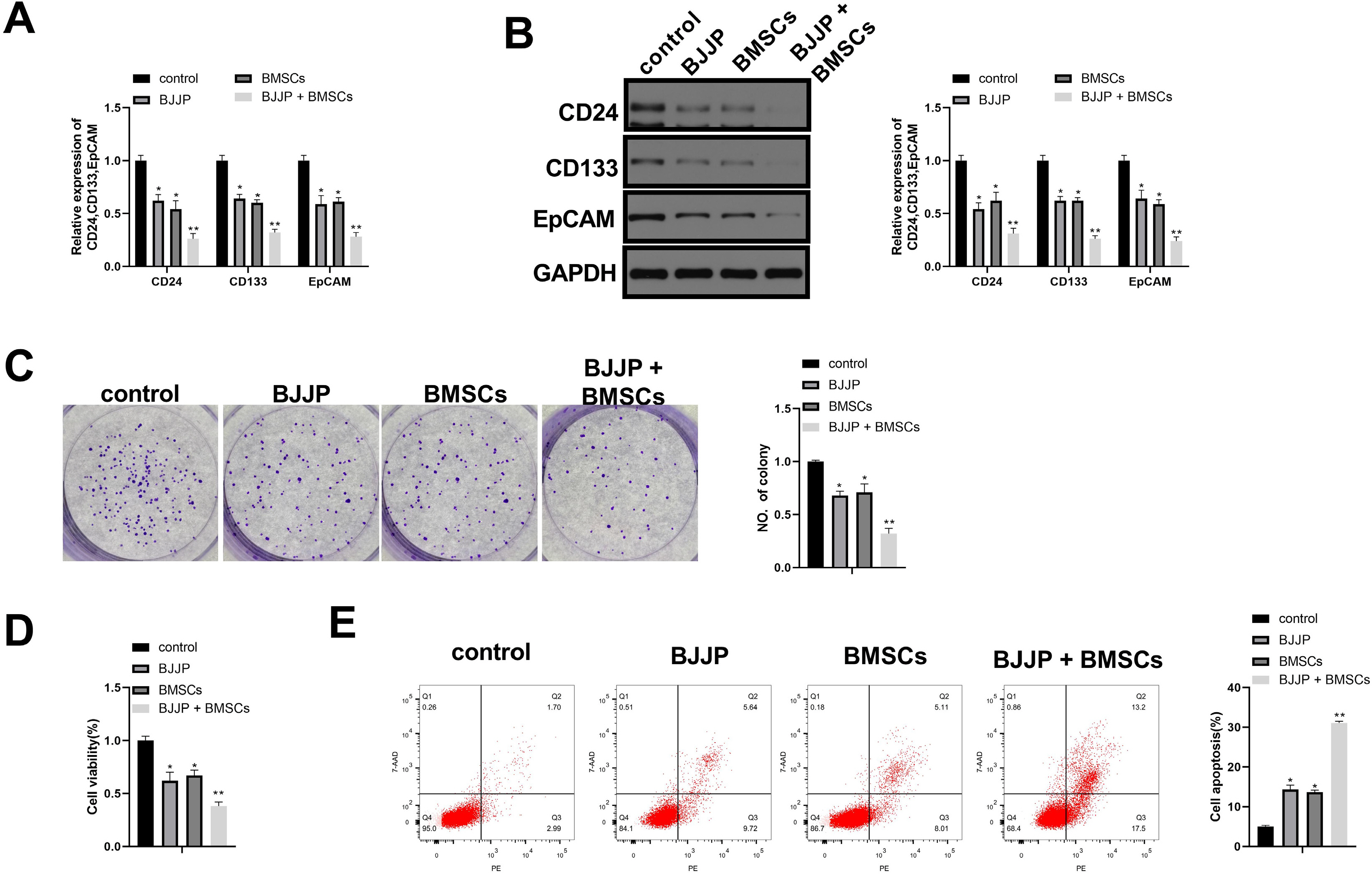
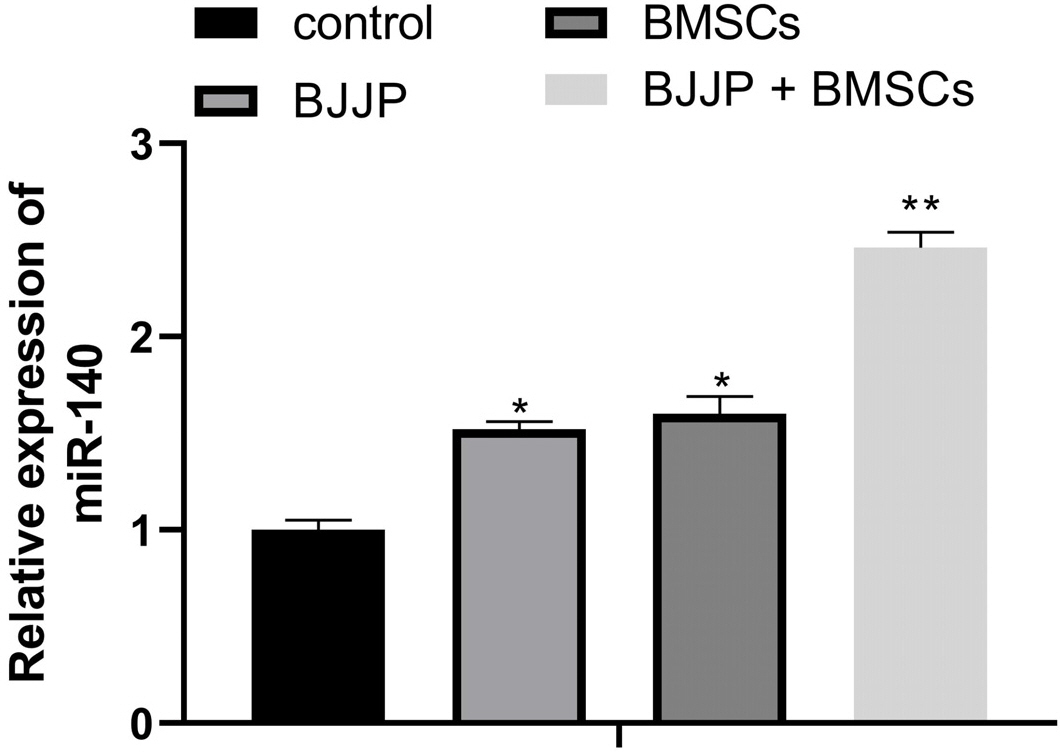
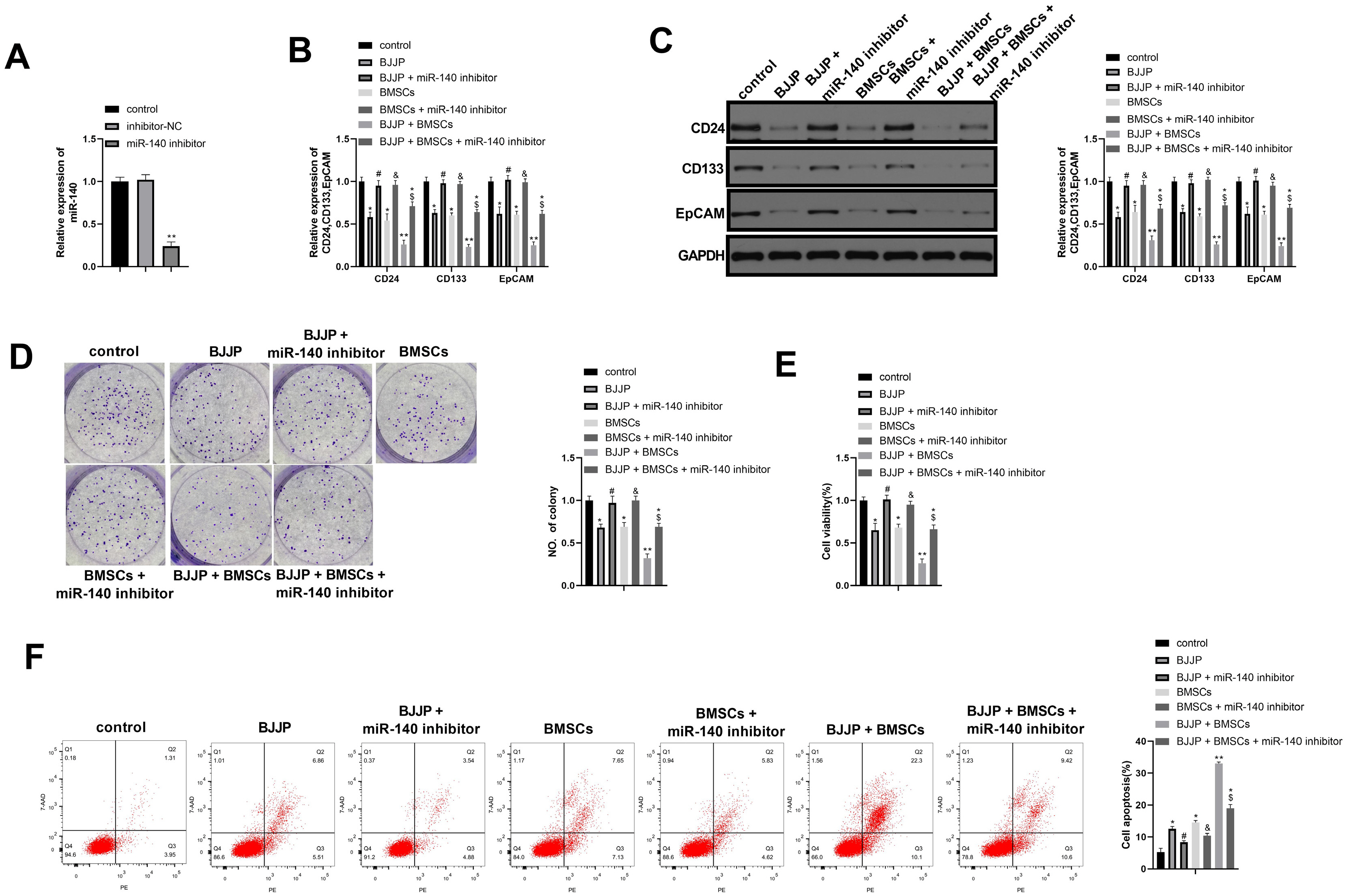
Flow cytometry was used to identify BMSCs isolated from BALB/c mice and CSCs enriched from Huh7 cells by measuring CD24, CD133, CD44, CD73, CD105, CD166, CD29, CD14 and CD34. Differentiation potential of BMSCs was also determined. Cell viability and proliferation ability of CSCs were determined by CCK-8 assay and clone formation assay. The expressions of CSCs biomarkers and Wnt/β-catenin signal pathway related proteins were determined by PCR and western blot. TOP-Flash/FOP-Flash luciferase assay was applied to measure the activity of β-catenin/TCF. Compared with untreated CSCs, BJJP or BMSCs treatment alone on CSCs lead to increased miR-140 expression and cell apoptosis, as well as decreased expressions of CD24, CD133, EpCAM and cell viability. Downregualted expressions of Wnt/β-catenin signal pathway related proteins, Wnt3a and β-catenin were found in response to BJJP or BMSCs treatment alone. The combination of BJJP+BMSCs treatment on CSCs could further enhance the suppressive effect on CSCs. Down-regulation of miR-140 in CSCs partially blocked the effects of BMSCs or BMSCs+BJJP on the expressions of Wnt3a and β-catenin as well as the cell viability and apoptosis of CSCs. Reversed expression pattern was found in CSCs transfected with miR-140 overexpression.
Conclusions
Taken together, we demonstrate that BJJP+BMSCs together could further enhance the suppressive effect on CSCs through regulating miR-140 and suppressing Wnt/β-catenin signal pathway. This study demonstrated the potential of BJJP+BMSCs in therapeutic treatment of HCC.
Keyword
Figure
Reference
-
References
1. Wu JZ, Jiang N, Lin JM, Liu X. 2020; STYXL1 promotes malignant progression of hepatocellular carcinoma via downregulating CELF2 through the PI3K/Akt pathway. Eur Rev Med Pharmacol Sci. 24:2977–2985. DOI: 10.26355/eurrev_202003_20662. PMID: 32271415.2. Ma W, Zhu D, Li J, Chen X, Xie W, Jiang X, Wu L, Wang G, Xiao Y, Liu Z, Wang F, Li A, Shao D, Dong W, Liu W, Yuan Y. 2020; Coating biomimetic nanoparticles with chimeric antigen receptor T cell-membrane provides high specificity for hepatocellular carcinoma photothermal therapy treatment. Theranostics. 10:1281–1295. DOI: 10.7150/thno.40291. PMID: 31938065. PMCID: PMC6956810.
Article3. El-Serag HB. 2011; Hepatocellular carcinoma. N Engl J Med. 365:1118–1127. DOI: 10.1056/NEJMra1001683. PMID: 21992124.
Article4. Shao Y, Song X, Jiang W, Chen Y, Ning Z, Gu W, Jiang J. 2019; MicroRNA-621 acts as a tumor radiosensitizer by directly targeting SETDB1 in hepatocellular carcinoma. Mol Ther. 27:355–364. DOI: 10.1016/j.ymthe.2018.11.005. PMID: 30503969. PMCID: PMC6369664.
Article5. Padthaisong S, Thanee M, Namwat N, Phetcharaburanin J, Klanrit P, Khuntikeo N, Titapun A, Sungkhamanon S, Saya H, Loilome W. 2020; Overexpression of a panel of cancer stem cell markers enhances the predictive capability of the progression and recurrence in the early stage cholangio-carcinoma. J Transl Med. 18:64. DOI: 10.1186/s12967-020-02243-w. PMID: 32039729. PMCID: PMC7008521.
Article6. Wu Y, Zhang J, Zhang X, Zhou H, Liu G, Li Q. 2020; Cancer stem cells: a potential breakthrough in HCC-targeted therapy. Front Pharmacol. 11:198. DOI: 10.3389/fphar.2020.00198. PMID: 32210805. PMCID: PMC7068598.
Article7. Rada T, Santos TC, Marques AP, Correlo VM, Frias AM, Castro AG, Neves NM, Gomes ME, Reis RL. 2012; Osteogenic differentiation of two distinct subpopulations of human adipose-derived stem cells: an in vitro and in vivo study. J Tissue Eng Regen Med. 6:1–11. DOI: 10.1002/term.388. PMID: 21294275.
Article8. Hong IS, Lee HY, Kang KS. 2014; Mesenchymal stem cells and cancer: friends or enemies? Mutat Res. 768:98–106. DOI: 10.1016/j.mrfmmm.2014.01.006. PMID: 24512984.
Article9. Yagi H, Kitagawa Y. 2013; The role of mesenchymal stem cells in cancer development. Front Genet. 4:261. DOI: 10.3389/fgene.2013.00261. PMID: 24348516. PMCID: PMC3842093.
Article10. Zhang Y, Shi K, Liu H, Chen W, Luo Y, Wei X, Wu Z. 2020; miR-4458 inhibits the epithelial-mesenchymal transition of hepatocellular carcinoma cells by suppressing the TGF-β signaling pathway via targeting TGFBR1. Acta Biochim Biophys Sin (Shanghai). 52:554–562. DOI: 10.1093/abbs/gmaa029. PMID: 32324847.
Article11. Yang H, Fang F, Chang R, Yang L. 2013; MicroRNA-140-5p suppresses tumor growth and metastasis by targeting transforming growth factor β receptor 1 and fibroblast growth factor 9 in hepatocellular carcinoma. Hepatology. 58:205–217. DOI: 10.1002/hep.26315. PMID: 23401231.
Article12. Takata A, Otsuka M, Yoshikawa T, Kishikawa T, Hikiba Y, Obi S, Goto T, Kang YJ, Maeda S, Yoshida H, Omata M, Asahara H, Koike K. 2013; MicroRNA-140 acts as a liver tumor suppressor by controlling NF-κB activity by directly targeting DNA methyltransferase 1 (Dnmt1) expression. Hepatology. 57:162–170. DOI: 10.1002/hep.26011. PMID: 22898998. PMCID: PMC3521841.
Article13. Lu Z, Yu Y, Ding X, Jin D, Wang G, Zhou Y, Zhu Y, Na L, He Y, Wang Q. 2020; LncRNA FLJ33360 accelerates the metastasis in hepatocellular carcinoma by targeting miRNA-140/MMP9 axis. Am J Transl Res. 12:583–591. PMID: 32194906. PMCID: PMC7061837.14. Mahboudi H, Soleimani M, Hanaee-Ahvaz H, Ghanbarian H, Bandehpour M, Enderami SE, Kazemi B. 2018; New approach for differentiation of bone marrow mesenchymal stem cells toward chondrocyte cells with overexpression of MicroRNA-140. ASAIO J. 64:662–672. DOI: 10.1097/MAT.0000000000000688. PMID: 29040159.
Article15. Liu X, Li M, Wang X, Dang Z, Yu L, Wang X, Jiang Y, Yang Z. 2019; Effects of adjuvant traditional Chinese medicine therapy on long-term survival in patients with hepato-cellular carcinoma. Phytomedicine. 62:152930. DOI: 10.1016/j.phymed.2019.152930. PMID: 31128485.
Article16. Jianxin C, Qingxia X, Junhui W, Qinhong Z. 2017; A case of recurrent hepatocellular carcinoma acquiring complete remission of target lesion with treatment with traditional Chinese medicine. Integr Cancer Ther. 16:597–604. DOI: 10.1177/1534735416660617. PMID: 27444311. PMCID: PMC5739135.
Article17. Zhang T, Yang Y, Wang B, Zheng X, Wang L, Feng X, Li G, Shi J, Cao N. 2019; Meta-analysis of influences of Biejiajian Pill combined with entecavir on serum liver fibrosis markers of compensatory period of hepatitis b cirrhosis: protocol of systematic review and meta-analysis. Medicine (Baltimore). 98:e18458. DOI: 10.1097/MD.0000000000018458. PMID: 31861022. PMCID: PMC6940166.18. Chang XF, Song CH, Chen T, Chen HY, Han LM. 2015; Effect of BieJiaJianWan on human hepatocellular carcinoma cells in Bel-7402 through MAPK signal pathway. J Gannan Med Coll. 35:663–666.19. Affo S, Yu LX, Schwabe RF. 2017; The role of cancer-associated fibroblasts and fibrosis in liver cancer. Annu Rev Pathol. 12:153–186. DOI: 10.1146/annurev-pathol-052016-100322. PMID: 27959632. PMCID: PMC5720358.
Article20. Serakinci N, Tulay P, Kalkan R. 2018; Role of mesenchymal stem cells in cancer development and their use in cancer therapy. Adv Exp Med Biol. 1083:45–62. DOI: 10.1007/5584_2017_64. PMID: 28730382.
Article21. Yoshida A, Kitajima S, Li F, Cheng C, Takegami Y, Kohno S, Wan YS, Hayashi N, Muranaka H, Nishimoto Y, Nagatani N, Nishiuchi T, Thai TC, Suzuki S, Nakao S, Tanaka T, Hirose O, Barbie DA, Takahashi C. 2017; MicroRNA-140 mediates RB tumor suppressor function to control stem cell-like activity through interleukin-6. Oncotarget. 8:13872–13885. DOI: 10.18632/oncotarget.14681. PMID: 28099924. PMCID: PMC5355146.
Article22. Wu D, Zhang J, Lu Y, Bo S, Li L, Wang L, Zhang Q, Mao J. 2019; miR-140-5p inhibits the proliferation and enhances the efficacy of doxorubicin to breast cancer stem cells by targeting Wnt1. Cancer Gene Ther. 26:74–82. DOI: 10.1038/s41417-018-0035-0. PMID: 30032164.
Article23. Reya T, Clevers H. 2005; Wnt signalling in stem cells and cancer. Nature. 434:843–850. DOI: 10.1038/nature03319. PMID: 15829953.
Article24. Hou J, Zhao N, Zhu P, Chang J, Du Y, Shen W. 2020; Irradiated mesenchymal stem cells support stemness maintenance of hepatocellular carcinoma stem cells through Wnt/β-ca-tenin signaling pathway. Cell Biosci. 10:93. DOI: 10.1186/s13578-020-00449-5. PMID: 32774840. PMCID: PMC7398068.
Article25. Liu D, Hong Y, Li Y, Hu C, Yip TC, Yu WK, Zhu Y, Fong CC, Wang W, Au SK, Wang S, Yang M. 2020; Targeted destruction of cancer stem cells using multifunctional magnetic nanoparticles that enable combined hyperthermia and chemotherapy. Theranostics. 10:1181–1196. DOI: 10.7150/thno.38989. PMID: 31938059. PMCID: PMC6956796.
Article26. Acikgoz E, Tatar C, Oktem G. 2020; Triptolide inhibits CD133+/CD44+ colon cancer stem cell growth and migration through triggering apoptosis and represses epithelial-mesenchymal transition via downregulating expressions of snail, slug, and twist. J Cell Biochem. 121:3313–3324. DOI: 10.1002/jcb.29602. PMID: 31904143.
Article27. Lin Q, Wu Z, Yue X, Yu X, Wang Z, Song X, Xu L, He Y, Ge Y, Tan S, Wang T, Song H, Yuan D, Gong Y, Gao L, Liang X, Ma C. 2020; ZHX2 restricts hepatocellular carcinoma by suppressing stem cell-like traits through KDM2A-mediated H3K36 demethylation. EBioMedicine. 53:102676. DOI: 10.1016/j.ebiom.2020.102676. PMID: 32114388. PMCID: PMC7047184.
Article28. Park DJ, Sung PS, Kim JH, Lee GW, Jang JW, Jung ES, Bae SH, Choi JY, Yoon SK. 2020; EpCAM-high liver cancer stem cells resist natural killer cell-mediated cytotoxicity by upregulating CEACAM1. J Immunother Cancer. 8:e000301. DOI: 10.1136/jitc-2019-000301. PMID: 32221015. PMCID: PMC7206970.
Article29. Wang ZC, Yang S, Huang JJ, Chen SL, Li QQ, Li Y. 2014; Effect of bone marrow mesenchymal stem cells on the Smad expression of hepatic fibrosis rats. Asian Pac J Trop Med. 7:321–324. DOI: 10.1016/S1995-7645(14)60048-1. PMID: 24507685.
Article30. Schwartz RE, Reyes M, Koodie L, Jiang Y, Blackstad M, Lund T, Lenvik T, Johnson S, Hu WS, Verfaillie CM. 2002; Multipotent adult progenitor cells from bone marrow differentiate into functional hepatocyte-like cells. J Clin Invest. 109:1291–1302. DOI: 10.1172/JCI0215182. PMID: 12021244. PMCID: PMC150983.
Article31. Khakoo AY, Pati S, Anderson SA, Reid W, Elshal MF, Rovira II, Nguyen AT, Malide D, Combs CA, Hall G, Zhang J, Raffeld M, Rogers TB, Stetler-Stevenson W, Frank JA, Reitz M, Finkel T. 2006; Human mesenchymal stem cells exert potent antitumorigenic effects in a model of Kaposi's sarcoma. J Exp Med. 203:1235–1247. DOI: 10.1084/jem.20051921. PMID: 16636132. PMCID: PMC2121206.
Article32. Wang ZC, Yang S, Huang JJ, Chen SL, Li QQ, Li Y. 2014; Effect of Rougan Huaqian granules combined with human mesenchymal stem cell transplantation on liver fibrosis in cirrhosis rats. Asian Pac J Trop Med. 7:576–581. DOI: 10.1016/S1995-7645(14)60097-3. PMID: 25063290.
Article33. Shi S, Zhang M, Guo R, Miao Y, Zhang M, Hu J, Xi Y, Li B. 2014; Feasibility of lentiviral-mediated sodium iodide symporter gene delivery for the efficient monitoring of bone marrow-derived mesenchymal stem cell transplantation and survival. Int J Mol Med. 34:1547–1554. DOI: 10.3892/ijmm.2014.1970. PMID: 25319483.
Article34. Wen ZX, Huang JJ, Wang ZC. 2019; Research on the transformation of hepatocyte from bone marrow mesenchymal stem cells by Biejiajianwan drug serum. Guiding J Tradit Chin Med Pharm. 25:35–38.35. Yang S, Li X, Shen W, Hu H, Li C, Han G. 2021; MicroRNA-140 represses esophageal cancer progression via targeting ZEB2 to regulate Wnt/β-catenin pathway. J Surg Res. 257:267–277. DOI: 10.1016/j.jss.2020.07.074. PMID: 32862055.
Article36. Zhao G, Yin Y, Zhao B. 2020; miR-140-5p is negatively correlated with proliferation, invasion, and tumorigenesis in malignant melanoma by targeting SOX4 via the Wnt/β-catenin and NF-κB cascades. J Cell Physiol. 235:2161–2170. DOI: 10.1002/jcp.29122. PMID: 31385607.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Use of Mesenchymal Stem Cells in Bone Regeneration
- Cellular origin of liver cancer stem cells
- Concise Review: Differentiation of Human Adult Stem Cells Into Hepatocyte-like Cells In vitro
- Role of Mesenchymal Stem Cells in Patients with Nontraumatic Osteonecrosis of the Femoral Head
- Stem Cells and Niemann Pick Disease