Nutr Res Pract.
2021 Aug;15(4):444-455. 10.4162/nrp.2021.15.4.444.
Effects of quercetin on cell differentiation and adipogenesis in 3T3-L1 adipocytes
- Affiliations
-
- 1Department of Food Science and Nutrition, Dankook University, Cheonan 31116, Korea
- 2Natural Nutraceuticals Industrialization Research Center, Dankook University, Cheonan 31116, Korea
- KMID: 2518753
- DOI: http://doi.org/10.4162/nrp.2021.15.4.444
Abstract
- BACKGROUND/OBJECTIVES
Adipocytes undergo angiogenesis to receive nutrients and oxygen needed for adipocyte' growth and differentiation. No study relating quercetin with angiogenesis in adipocytes exists. Therefore, this study investigated the role of quercetin on adipogenesis in 3T3-L1 cells, acting through matrix metalloproteinases (MMPs).
MATERIALS/METHODS
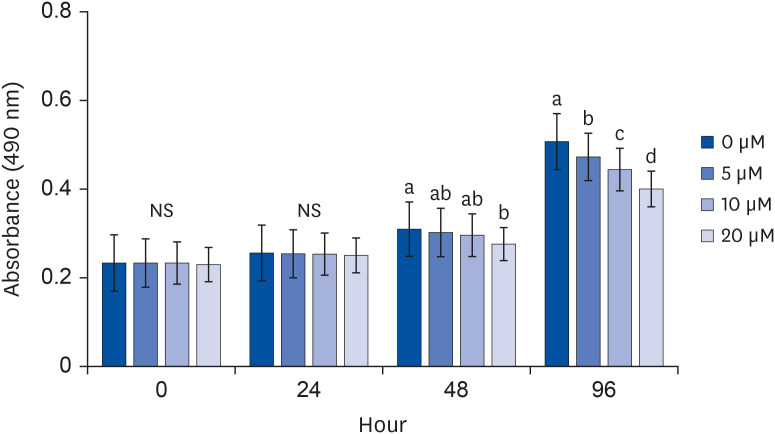
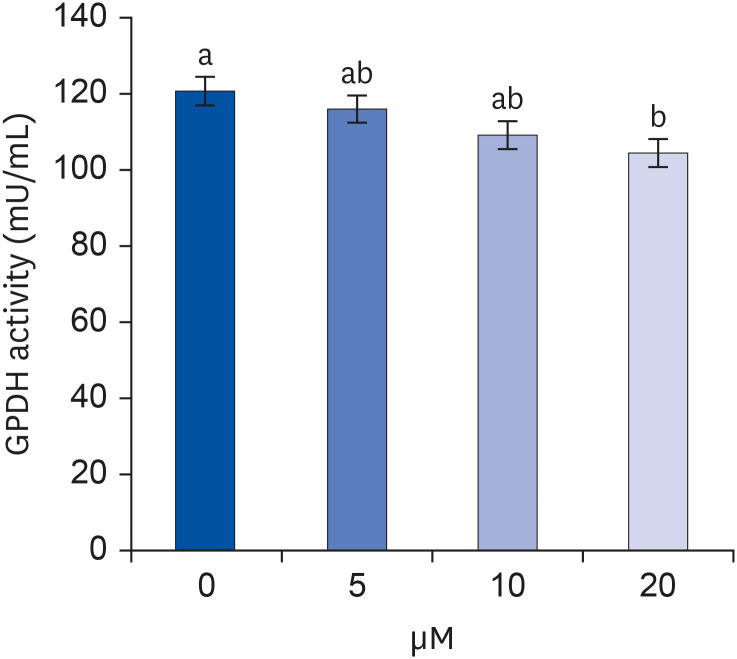
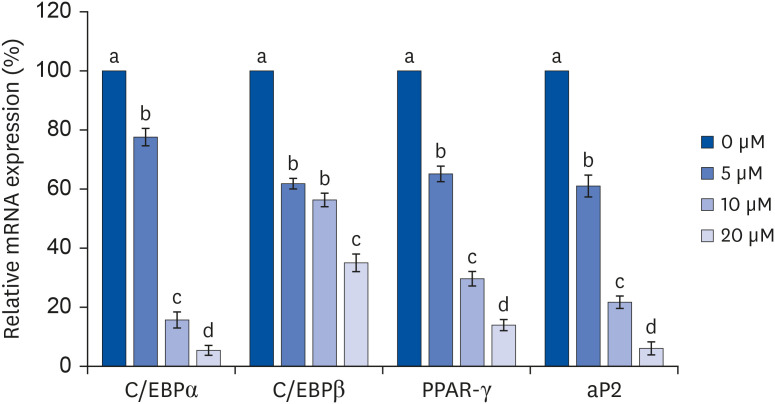
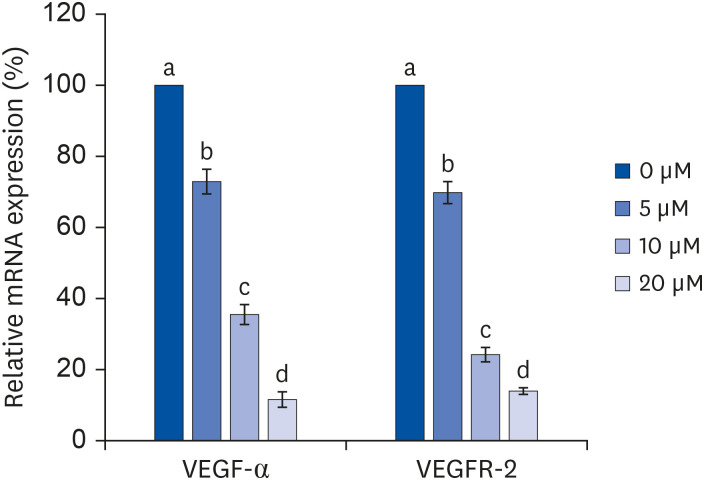
After proliferating preadipocytes into adipocytes, various quercetin concentrations were added to adipocytes, and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assays were performed to evaluate cell proliferation. Glycerol-3-phosphate dehydrogenase (GPDH) activity was investigated as an indicator of fat accumulation. The mRNA expressions of transcription factors related to adipocyte differentiation, CCAAT/enhancer-binding proteins (C/EBPs), peroxisomal proliferatoractivated receptors (PPAR)-γ, and adipocyte protein 2 (aP2), were investigated. The mRNA expressions of proteins related to angiogenesis, vascular endothelial growth factor (VEGF)-α, vascular endothelial growth factor receptor (VEGFR)-2, MMP-2, and MMP-9, were investigated. Enzyme activities and concentrations of MMP-2 and MMP-9 were also measured.
RESULTS
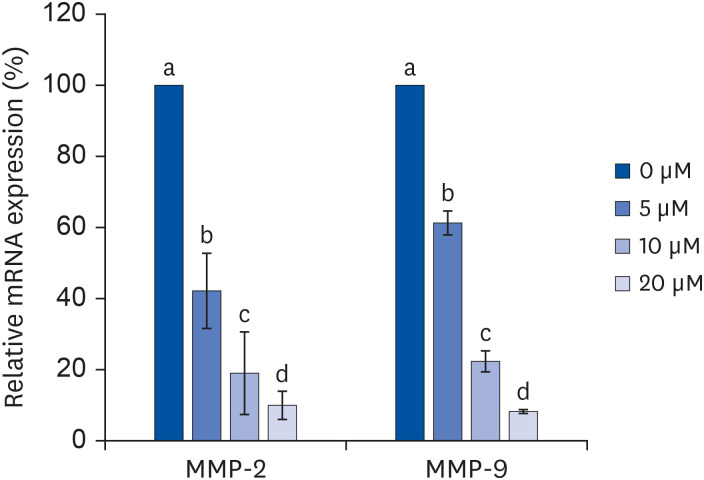
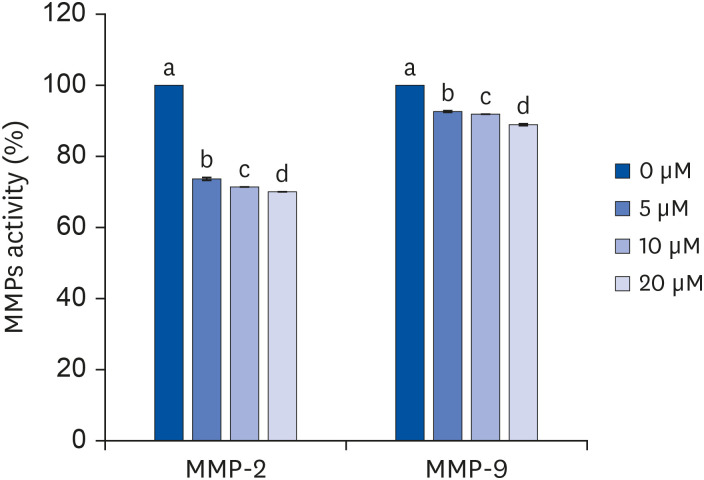
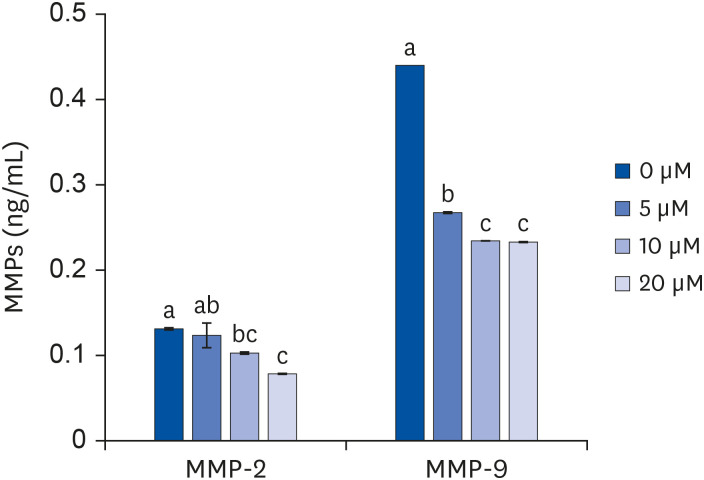
Quercetin treatment suppressed fat accumulation and the expressions of adipocyte differentiation-related genes (C/EBPα, C/EBPβ, PPAR-γ, and aP2) in a concentration-dependent manner in 3T3-L1 cells. Quercetin treatments reduced the mRNA expressions of VEGF-α, VEGFR-2, MMP-2, and MMP-9 in 3T3-L1 cells. The activities and concentrations of MMP-2 and MMP-9 were also decreased significantly as the concentration of quercetin increased.
CONCLUSIONS
The results confirm that quercetin inhibits adipose tissue differentiation and fat accumulation in 3T3-L1 cells, which could occur through inhibition of the angiogenesis process related to MMPs.
Figure
Reference
-
2. Cao Y. Angiogenesis modulates adipogenesis and obesity. J Clin Invest. 2007; 117:2362–2368. PMID: 17786229.
Article3. Ali AT, Hochfeld WE, Myburgh R, Pepper MS. Adipocyte and adipogenesis. Eur J Cell Biol. 2013; 92:229–236. PMID: 23876739.
Article4. Almalki SG, Llamas Valle Y, Agrawal DK. MMP-2 and MMP-14 silencing inhibits VEGFR2 cleavage and induces the differentiation of porcine adipose-derived mesenchymal stem cells to endothelial cells. Stem Cells Transl Med. 2017; 6:1385–1398. PMID: 28213979.
Article5. Bagchi M, Kim LA, Boucher J, Walshe TE, Kahn CR, D'Amore PA. Vascular endothelial growth factor is important for brown adipose tissue development and maintenance. FASEB J. 2013; 27:3257–3271. PMID: 23682123.
Article6. Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003; 9:669–676. PMID: 12778165.
Article7. Berg G, Barchuk M, Miksztowicz V. Behavior of metalloproteinases in adipose tissue, liver and arterial wall: an update of extracellular matrix remodeling. Cells. 2019; 8:158.
Article8. Carullo G, Cappello AR, Frattaruolo L, Badolato M, Armentano B, Aiello F. Quercetin and derivatives: useful tools in inflammation and pain management. Future Med Chem. 2017; 9:79–93. PMID: 27995808.
Article9. Chen S, Jiang H, Wu X, Fang J. Therapeutic effects of quercetin on inflammation, obesity, and type 2 diabetes. Mediators Inflamm. 2016; 2016:9340637. PMID: 28003714.
Article10. Zhao Y, Chen B, Shen J, Wan L, Zhu Y, Yi T, Xiao Z. The beneficial effects of quercetin, curcumin, and resveratrol in obesity. Oxid Med Cell Longev. 2017; 2017:1459497. PMID: 29138673.
Article11. Ahn J, Lee H, Kim S, Park J, Ha T. The anti-obesity effect of quercetin is mediated by the AMPK and MAPK signaling pathways. Biochem Biophys Res Commun. 2008; 373:545–549. PMID: 18586010.
Article12. Bae CR, Park YK, Cha YS. Quercetin-rich onion peel extract suppresses adipogenesis by down-regulating adipogenic transcription factors and gene expression in 3T3-L1 adipocytes. J Sci Food Agric. 2014; 94:2655–2660. PMID: 24634340.
Article13. Lu J, Wang Z, Li S, Xin Q, Yuan M, Li H, Song X, Gao H, Pervaiz N, Sun X, et al. Quercetin inhibits the migration and invasion of hcclm3 cells by suppressing the expression of p-Akt1, matrix metalloproteinase (MMP) MMP-2, and MMP-9. Med Sci Monit. 2018; 24:2583–2589. PMID: 29701200.
Article14. Ren KW, Li YH, Wu G, Ren JZ, Lu HB, Li ZM, Han XW. Quercetin nanoparticles display antitumor activity via proliferation inhibition and apoptosis induction in liver cancer cells. Int J Oncol. 2017; 50:1299–1311. PMID: 28259895.
Article15. Chuang CH, Yeh CL, Yeh SL, Lin ES, Wang LY, Wang YH. Quercetin metabolites inhibit MMP-2 expression in A549 lung cancer cells by PPAR-γ associated mechanisms. J Nutr Biochem. 2016; 33:45–53. PMID: 27260467.
Article16. Zhao J, Fang Z, Zha Z, Sun Q, Wang H, Sun M, Qiao B. Quercetin inhibits cell viability, migration and invasion by regulating miR-16/HOXA10 axis in oral cancer. Eur J Pharmacol. 2019; 847:11–18. PMID: 30639311.
Article17. Kim WK, Kang NE, Kim MH, Ha AW. Peanut sprout ethanol extract inhibits the adipocyte proliferation, differentiation, and matrix metalloproteinases activities in mouse fibroblast 3T3-L1 preadipocytes. Nutr Res Pract. 2013; 7:160–165. PMID: 23766875.
Article18. Kim HL, Ha AW, Kim WK. Effect of saccharin on inflammation in 3T3-L1 adipocytes and the related mechanism. Nutr Res Pract. 2020; 14:109–116. PMID: 32256985.
Article19. Harwood M, Danielewska-Nikiel B, Borzelleca JF, Flamm GW, Williams GM, Lines TC. A critical review of the data related to the safety of quercetin and lack of evidence of in vivo toxicity, including lack of genotoxic/carcinogenic properties. Food Chem Toxicol. 2007; 45:2179–2205. PMID: 17698276.20. Eseberri I, Miranda J, Lasa A, Churruca I, Portillo MP. Doses of quercetin in the range of serum concentrations exert delipidating effects in 3T3-L1 preadipocytes by acting on different stages of adipogenesis, but not in mature adipocytes. Oxid Med Cell Longev. 2015; 2015:480943. PMID: 26180590.
Article21. Lee CW, Seo JY, Lee J, Choi JW, Cho S, Bae JY, Sohng JK, Kim SO, Kim J, Park YI. 3-O-Glucosylation of quercetin enhances inhibitory effects on the adipocyte differentiation and lipogenesis. Biomed Pharmacother. 2017; 95:589–598. PMID: 28869898.
Article22. Yang JY, Della-Fera MA, Rayalam S, Ambati S, Hartzell DL, Park HJ, Baile CA. Enhanced inhibition of adipogenesis and induction of apoptosis in 3T3-L1 adipocytes with combinations of resveratrol and quercetin. Life Sci. 2008; 82:1032–1039. PMID: 18433793.
Article23. Lee MS, Kim Y. Effects of isorhamnetin on adipocyte mitochondrial biogenesis and AMPK activation. Molecules. 2018; 23:1853.
Article24. Lefterova MI, Zhang Y, Steger DJ, Schupp M, Schug J, Cristancho A, Feng D, Zhuo D, Stoeckert CJ Jr, Liu XS, et al. PPARγ and C/EBP factors orchestrate adipocyte biology via adjacent binding on a genome-wide scale. Genes Dev. 2008; 22:2941–2952. PMID: 18981473.
Article25. Kawamura T, Murakami K, Bujo H, Unoki H, Jiang M, Nakayama T, Saito Y. Matrix metalloproteinase-3 enhances the free fatty acids-induced VEGF expression in adipocytes through toll-like receptor 2. Exp Biol Med (Maywood). 2008; 233:1213–1221. PMID: 18641052.
Article26. Park BY, Lee H, Woo S, Yoon M, Kim J, Hong Y, Lee HS, Park EK, Hahm JC, Kim JW, et al. Reduction of adipose tissue mass by the angiogenesis inhibitor ALS-L1023 from Melissa officinalis. PLoS One. 2015; 10:e0141612. PMID: 26599360.
Article27. Chavey C, Mari B, Monthouel MN, Bonnafous S, Anglard P, Van Obberghen E, Tartare-Deckert S. Matrix metalloproteinases are differentially expressed in adipose tissue during obesity and modulate adipocyte differentiation. J Biol Chem. 2003; 278:11888–11896. PMID: 12529376.
Article28. Maquoi E, Munaut C, Colige A, Collen D, Lijnen HR. Modulation of adipose tissue expression of murine matrix metalloproteinases and their tissue inhibitors with obesity. Diabetes. 2002; 51:1093–1101. PMID: 11916931.
Article29. Pratheeshkumar P, Budhraja A, Son YO, Wang X, Zhang Z, Ding S, Wang L, Hitron A, Lee JC, Xu M, et al. Quercetin inhibits angiogenesis mediated human prostate tumor growth by targeting VEGFR-2 regulated AKT/mTOR/P70S6K signaling pathways. PLoS One. 2012; 7:e47516. PMID: 23094058.30. Bouloumié A, Sengenès C, Portolan G, Galitzky J, Lafontan M. Adipocyte produces matrix metalloproteinases 2 and 9: involvement in adipose differentiation. Diabetes. 2001; 50:2080–2086. PMID: 11522674.31. Andres S, Pevny S, Ziegenhagen R, Bakhiya N, Schäfer B, Hirsch-Ernst KI, Lampen A. Safety aspects of the use of quercetin as a dietary supplement. Mol Nutr Food Res. 2018; 62:1700447.
Article32. Conquer JA, Maiani G, Azzini E, Raguzzini A, Holub BJ. Supplementation with quercetin markedly increases plasma quercetin concentration without effect on selected risk factors for heart disease in healthy subjects. J Nutr. 1998; 128:593–597. PMID: 9482769.
Article33. Carbonaro M, Grant G. Absorption of quercetin and rutin in rat small intestine. Ann Nutr Metab. 2005; 49:178–182. PMID: 16006787.
Article34. Egert S, Bosy-Westphal A, Seiberl J, Kürbitz C, Settler U, Plachta-Danielzik S, Wagner AE, Frank J, Schrezenmeir J, Rimbach G, et al. Quercetin reduces systolic blood pressure and plasma oxidised low-density lipoprotein concentrations in overweight subjects with a high-cardiovascular disease risk phenotype: a double-blinded, placebo-controlled cross-over study. Br J Nutr. 2009; 102:1065–1074. PMID: 19402938.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effects of Epigallocatechin on Adipogenesis of 3T3-L1 Preadipocytes
- Pear pomace water extract inhibits adipogenesis and induces apoptosis in 3T3-L1 adipocytes
- Cryptotanshinone Inhibits Lipid Accumulation in Differentiating 3T3-L1 Preadipocytes by Down-regulating C/EBP-α, PPAR-γ, FAS, Perilipin A, and STAT-3
- Cryptotanshinone Inhibits Lipid Accumulation in Differentiating 3T3-L1 Preadipocytes by Down-regulating C/EBP-α, PPAR-γ, FAS, Perilipin A, and STAT-3
- Cryptotanshinone Inhibits Lipid Accumulation in Differentiating 3T3-L1 Preadipocytes by Down-regulating C/EBP-α, PPAR-γ, FAS, Perilipin A, and STAT-3