Intest Res.
2021 Jul;19(3):301-312. 10.5217/ir.2020.00013.
Is adalimumab safe and effective in patients with intestinal Behcet’s disease in real-world practice?
- Affiliations
-
- 1Department of Internal Medicine, Sakura Medical Center, Toho University, Chiba, Japan
- 2Medical Department, AbbVie GK, Tokyo, Japan
- 3Center for Advanced IBD Research and Treatment, Kitasato University Kitasato Institute Hospital, Tokyo, Japan
- KMID: 2518686
- DOI: http://doi.org/10.5217/ir.2020.00013
Abstract
- Background/Aims
The safety and effectiveness of adalimumab was demonstrated in a phase 3 trial in Japanese patients with intestinal Behçet’s disease. The aim of this study was to evaluate the long-term safety and effectiveness of adalimumab in Japanese patients with intestinal Behçet’s disease.
Methods
This prospective, all-case, post-marketing study was conducted at 254 centers in Japanese patients with intestinal Behçet’s disease receiving adalimumab. The primary endpoint was incidence of adverse drug reactions. Effectiveness endpoints included global improvement rating and change in C-reactive protein levels.
Results
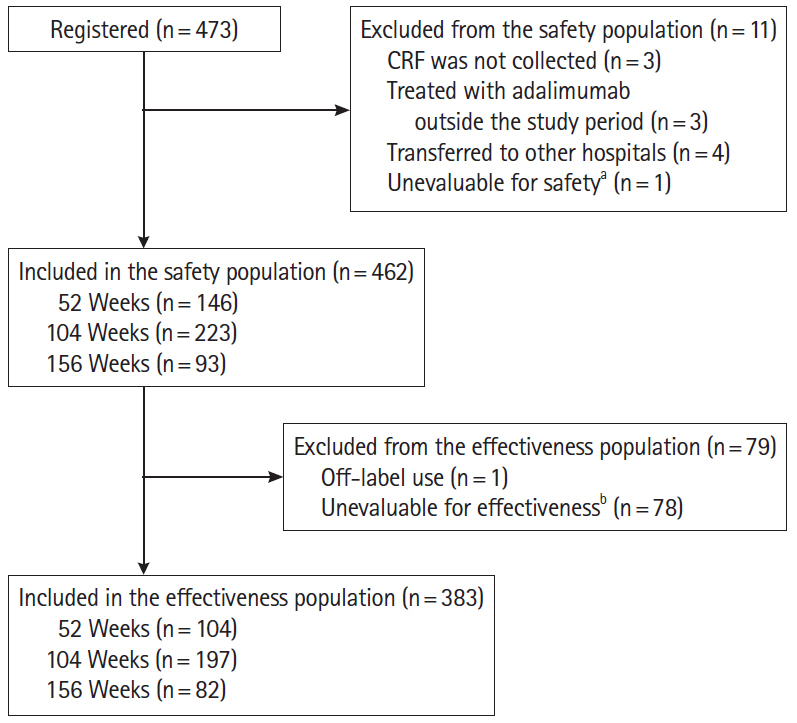
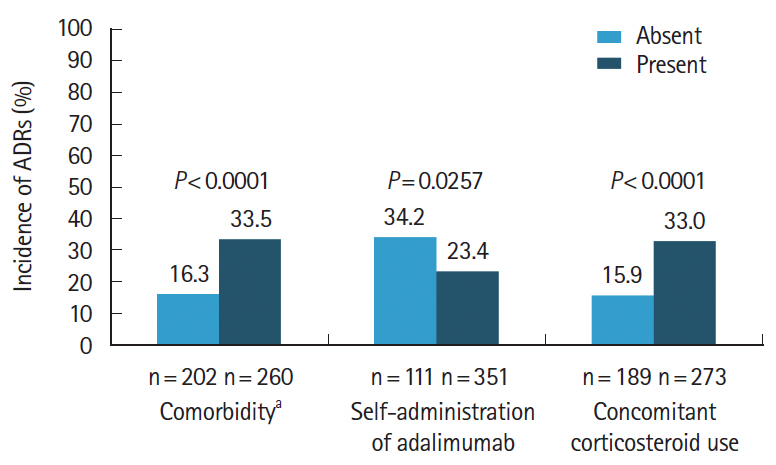
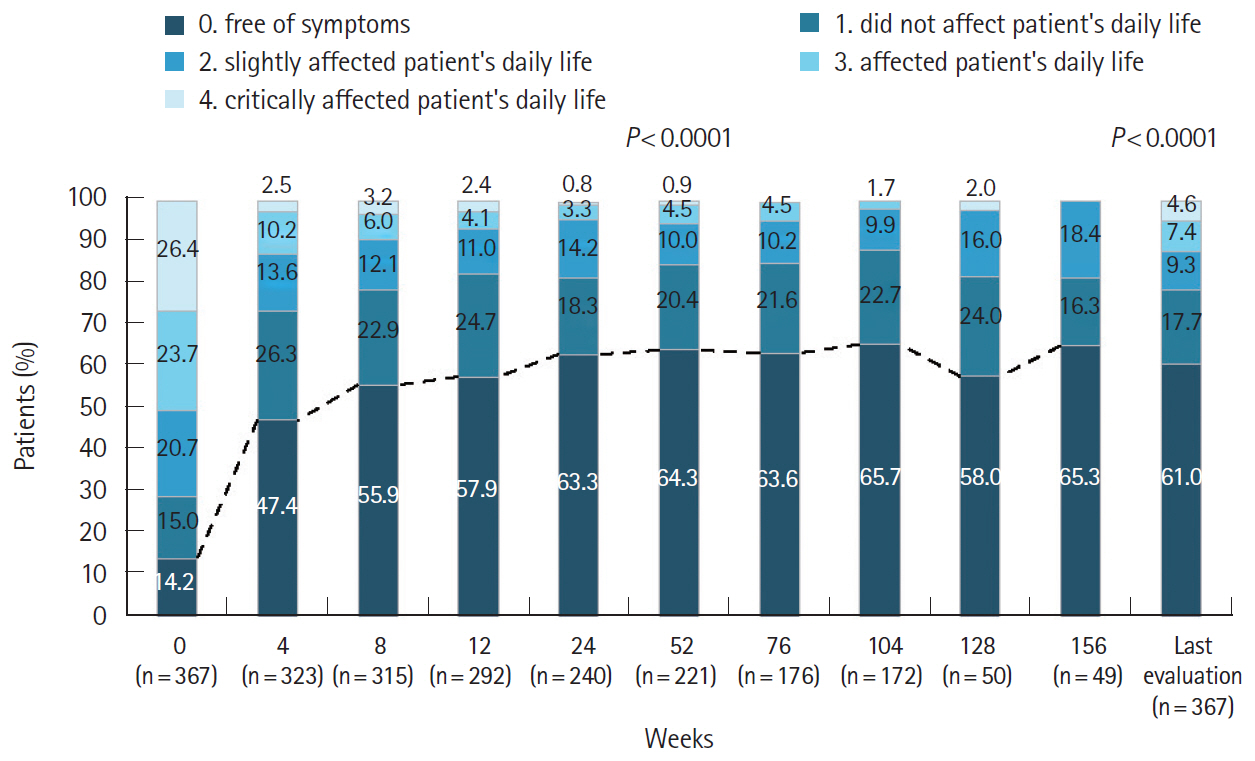
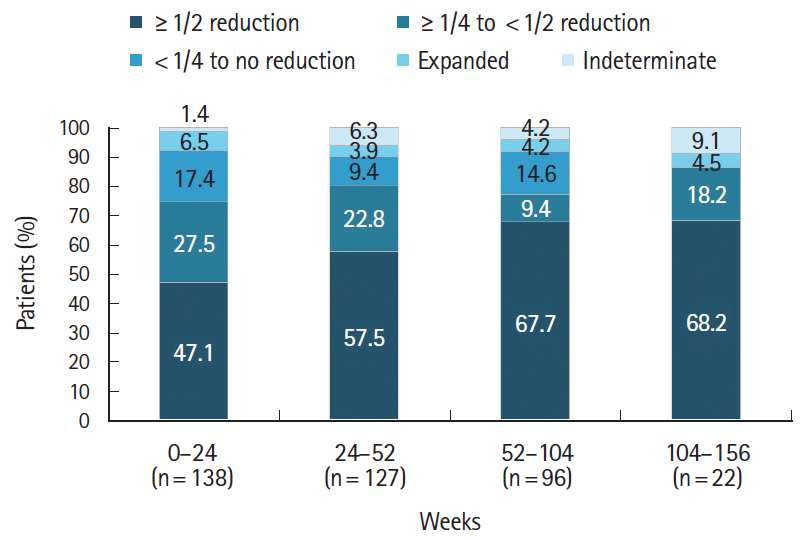
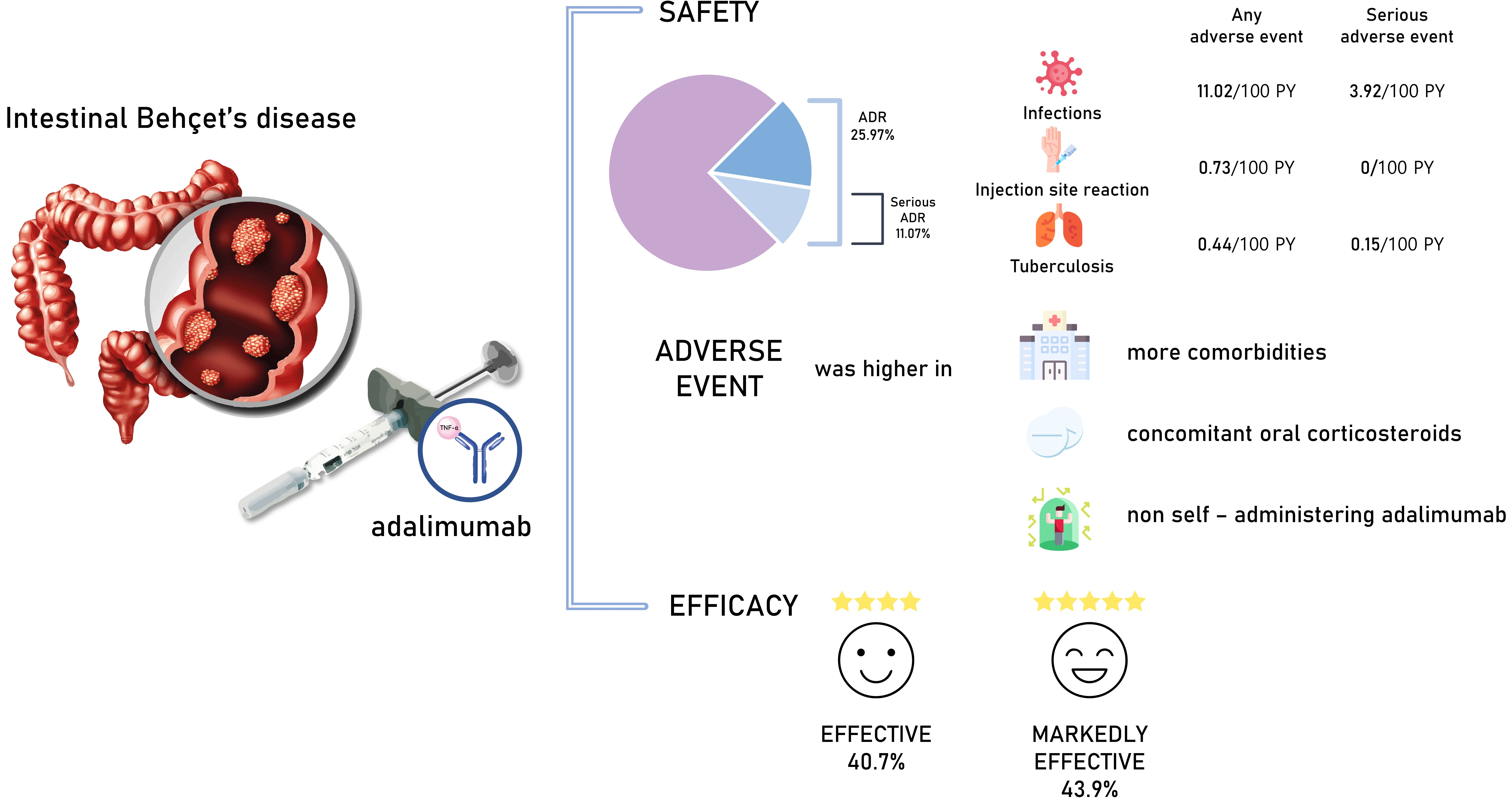
Of the 473 registered patients, 462 and 383 included in the safety and effectiveness populations were administered adalimumab for a mean of 515.3 and 579.5 days, respectively. Overall, 395 patients (85.5%) received adalimumab at the recommended dose. Adverse drug reactions and serious adverse drug reactions were reported in 120 (25.97%) and 51 (11.04%) patients, respectively. The incidence of adverse drug reactions was significantly higher in patients with comorbidities (P< 0.0001), patients taking concomitant oral corticosteroids (P< 0.0001), and those not self-administering adalimumab (P= 0.0257). At study end, global improvement rating was “effective” (n = 156, 40.7%) or “markedly effective” (n = 168, 43.9%) in 324 patients (overall effective, 84.6%). Mean C-reactive protein levels (mg/dL) decreased from 1.96 at baseline (n = 324) to 0.58 at week 24 (n = 208) and 0.25 at week 156 (n = 37).
Conclusions
This large real-world study confirmed the long-term safety and effectiveness of adalimumab in patients with intestinal Behçet’s disease. No new safety concerns were identified. (Clinical trial registration number: NCT01960790)
Figure
Reference
-
1. Saadoun D, Wechsler B. Behçet’s disease. Orphanet J Rare Dis. 2012; 7:20.
Article2. Sakane T, Takeno M, Suzuki N, Inaba G. Behçet’s disease. N Engl J Med. 1999; 341:1284–1291.
Article3. Keino H, Okada AA. Behçet’s disease: global epidemiology of an Old Silk Road disease. Br J Ophthalmol. 2007; 91:1573–1574.4. Matsuda T. Epidemiology of Behçet’s disease. Guidebook for Behçet’s disease. Tokyo: Nihonigakukan;2002.5. Ministry of Health, Labour and Welfare. The diagnostic criteria for Behçet’s disease [Internet]. c2003 [cited 2018 Feb 6]. http://www.mhlw.go.jp/file/06-Seisakujouhou-10900000-Kenkoukyoku/0000089968.pdf.6. Inoue N, Kobayashi K, Naganuma M, et al. Long-term safety and efficacy of adalimumab for intestinal Behçet’s disease in the open label study following a phase 3 clinical trial. Intest Res. 2017; 15:395–401.
Article7. al-Dalaan AN, al Balaa SR, el Ramahi K, et al. Behçet’s disease in Saudi Arabia. J Rheumatol. 1994; 21:658–661.
Article8. Zouboulis CC, Kötter I, Djawari D, et al. Epidemiological features of Adamantiades-Behçet’s disease in Germany and in Europe. Yonsei Med J. 1997; 38:411–422.
Article9. Chen YC, Chang HW. Clinical characteristics of Behçet’s disease in southern Taiwan. J Microbiol Immunol Infect. 2001; 34:207–210.10. Wang LY, Zhao DB, Gu J, Dai SM. Clinical characteristics of Behçet’s disease in China. Rheumatol Int. 2010; 30:1191–1196.
Article11. Singal A, Chhabra N, Pandhi D, Rohatgi J. Behçet’s disease in India: a dermatological perspective. Indian J Dermatol Venereol Leprol. 2013; 79:199–204.
Article12. Tanida S, Inoue N, Kobayashi K, et al. Adalimumab for the treatment of Japanese patients with intestinal Behçet’s disease. Clin Gastroenterol Hepatol. 2015; 13:940–948.
Article13. Kastner DL. Intermittent and periodic arthritic syndromes. In : Koopman WJ, editor. Arthritis and allied conditions: a textbook of rheumatology. 13th ed. Baltimore: Williams & Wilkins;1997. p. 1279–1306.14. Shimizu T, Ehrlich GE, Inaba G, Hayashi K. Behçet disease (Behçet syndrome). Semin Arthritis Rheum. 1979; 8:223–260.
Article15. Ideguchi H, Suda A, Takeno M, Ueda A, Ohno S, Ishigatsubo Y. Behçet disease: evolution of clinical manifestations. Medicine (Baltimore). 2011; 90:125–132.16. Hisamatsu T, Ueno F, Matsumoto T, et al. The 2nd edition of consensus statements for the diagnosis and management of intestinal Behçet’s disease: indication of anti-TNFα monoclonal antibodies. J Gastroenterol. 2014; 49:156–162.
Article17. Cheon JH, Celik AF, Kim WH. Behçet’s disease: gastrointestinal involvement. In : Yazici Y, Yazici H, editors. Behçet’s syndrome. 1st ed. New York: Springer;2010. p. 165–188.18. Comarmond C, Wechsler B, Cacoub P, Saadoun D. Approaches to immunosuppression in Behçet’s disease. Immunotherapy. 2013; 5:743–754.
Article19. Cheon JH, Kim WH. An update on the diagnosis, treatment, and prognosis of intestinal Behçet’s disease. Curr Opin Rheumatol. 2015; 27:24–31.
Article20. Chung MJ, Cheon JH, Kim SU, et al. Response rates to medical treatments and long-term clinical outcomes of nonsurgical patients with intestinal Behçet disease. J Clin Gastroenterol. 2010; 44:e116–e122.
Article21. Hisamatsu T, Hayashida M. Treatment and outcomes: medical and surgical treatment for intestinal Behçet’s disease. Intest Res. 2017; 15:318–327.
Article22. Nakase H, Okazaki K, Kawanami C, et al. Therapeutic effects on intestinal Behçet’s disease of an intravenous drug delivery system using dexamethasone incorporated in lipid emulsion. J Gastroenterol Hepatol. 2001; 16:1306–1308.
Article23. Toda K, Shiratori Y, Yasuda M, et al. Therapeutic effect of intraarterial prednisolone injection in severe intestinal Behçet’s disease. J Gastroenterol. 2002; 37:844–848.
Article24. Yasuo M, Miyabayashi H, Okano T, Aoki H, Ichikawa K, Hirose Y. Successful treatment with corticosteroid in a case of Behçet’s syndrome with multiple esophageal ulcerations. Intern Med. 2003; 42:696–699.
Article25. Park JJ, Cheon JH, Moon CM, et al. Long-term clinical outcomes after the first course of corticosteroid therapy in patients with moderate to severe intestinal Behget’s disease. Gastroenterology. 2010; 138(5 Suppl 1):S698–S699.26. Ogata H, Watanabe M, Matsui T, et al. Safety of adalimumab and predictors of adverse events in 1693 Japanese patients with Crohn’s disease. J Crohns Colitis. 2016; 10:1033–1041.
Article27. Pharmaceuticals and Medical Devices Agency. The Pharmaceuticals and Medical Devices Agency annual report FY 2013 (April 2013–March 2014) [Internet]. [cited 2018 Feb 9]. https://www.pmda.go.jp/files/000203634.pdf.28. Park JJ, Kim WH, Cheon JH. Outcome predictors for intestinal Behçet’s disease. Yonsei Med J. 2013; 54:1084–1090.
Article29. Burmester GR, Landewé R, Genovese MC, et al. Adalimumab long-term safety: infections, vaccination response and pregnancy outcomes in patients with rheumatoid arthritis. Ann Rheum Dis. 2017; 76:414–417.
Article30. Schiff MH, Burmester GR, Kent JD, et al. Safety analyses of adalimumab (HUMIRA) in global clinical trials and US postmarketing surveillance of patients with rheumatoid arthritis. Ann Rheum Dis. 2006; 65:889–894.
Article31. Colombel JF, Sandborn WJ, Panaccione R, et al. Adalimumab safety in global clinical trials of patients with Crohn’s disease. Inflamm Bowel Dis. 2009; 15:1308–1319.
Article32. Kamm MA, Hanauer SB, Panaccione R, et al. Adalimumab sustains steroid-free remission after 3 years of therapy for Crohn’s disease. Aliment Pharmacol Ther. 2011; 34:306–317.
Article33. Lichtenstein GR, Feagan BG, Cohen RD, et al. Serious infections and mortality in association with therapies for Crohn’s disease: TREAT registry. Clin Gastroenterol Hepatol. 2006; 4:621–630.
Article34. Criteria for diagnosis of Behçet’s disease. International Study Group for Behçet’s Disease. Lancet. 1990; 335:1078–1080.35. Hirai F, Watanabe K, Matsumoto T, et al. Patients’ assessment of adalimumab self-injection for Crohn’s disease: a multicenter questionnaire survey (The PEARL Survey). Hepatogastroenterology. 2014; 61:1654–1660.36. Tanida S, Mizoshita T, Nishie H, et al. Long-term efficacy of adalimumab in patients with intestinal Behcet’s disease: eight consecutive cases. J Clin Med Res. 2016; 8:334–337.
Article37. Bawazeer A, Raffa LH, Nizamuddin SH. Clinical experience with adalimumab in the treatment of ocular Behçet disease. Ocul Immunol Inflamm. 2010; 18:226–232.
Article38. Fabiani C, Vitale A, Emmi G, et al. Efficacy and safety of adalimumab in Behçet’s disease-related uveitis: a multicenter retrospective observational study. Clin Rheumatol. 2017; 36:183–189.
Article39. Perra D, Alba MA, Callejas JL, et al. Adalimumab for the treatment of Behçet’s disease: experience in 19 patients. Rheumatology (Oxford). 2012; 51:1825–1831.
Article40. Löfberg R, Louis EV, Reinisch W, et al. Adalimumab produces clinical remission and reduces extraintestinal manifestations in Crohn’s disease: results from CARE. Inflamm Bowel Dis. 2012; 18:1–9.
Article41. Louis EJ, Reinisch W, Schwartz DA, et al. Adalimumab reduces extraintestinal manifestations in patients with Crohn’s disease: a pooled analysis of 11 clinical studies. Adv Ther. 2018; 35:563–576.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Update on the Treatment of Intestinal Behcet's Disease
- Anti-Tumor Necrosis Factor Therapy in Intestinal Behçet's Disease
- Real-world effectiveness and safety of adalimumab in Korean patients with intestinal Behcet’s disease: a Korean Association for the Study of Intestinal Diseases (KASID) multicenter study
- A Case of Intestinal Behcet's Disease Complicated Enterocutanous Fistula with a Good Response to Adalimumab
- Could adalimumab be used safely and effectively in intestinal Behçet's disease refractory to conventional therapy?