Yeungnam Univ J Med.
2021 Jul;38(3):251-257. 10.12701/yujm.2020.00878.
Successful treatment with vedolizumab in an adolescent with Crohn disease who had developed active pulmonary tuberculosis while receiving infliximab
- Affiliations
-
- 1Department of Pediatrics, School of Medicine, Kyungpook National University, Daegu, Korea
- 2Crohn’s and Colitis Association in Daegu-Gyeongbuk (CCAiD), Daegu, Korea
- KMID: 2518669
- DOI: http://doi.org/10.12701/yujm.2020.00878
Abstract
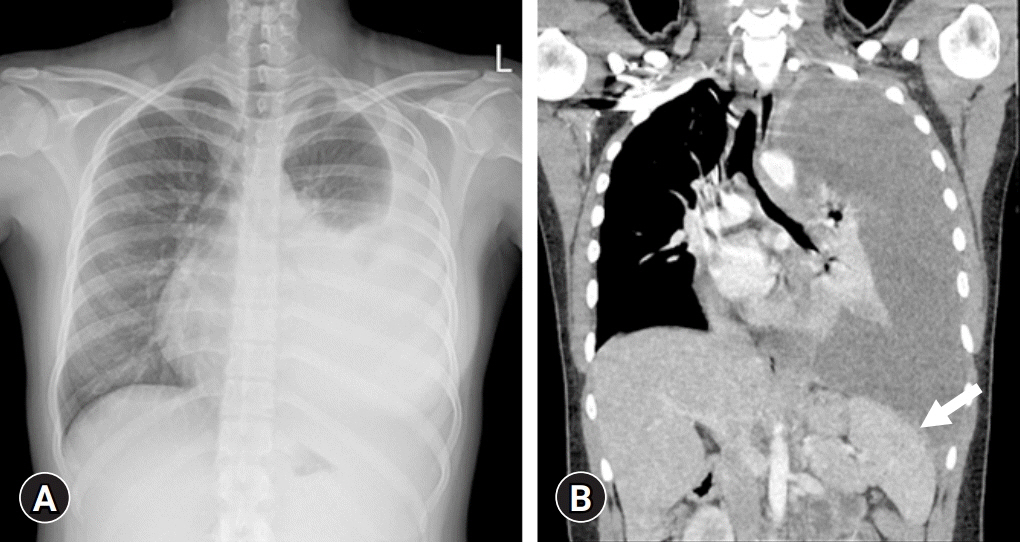
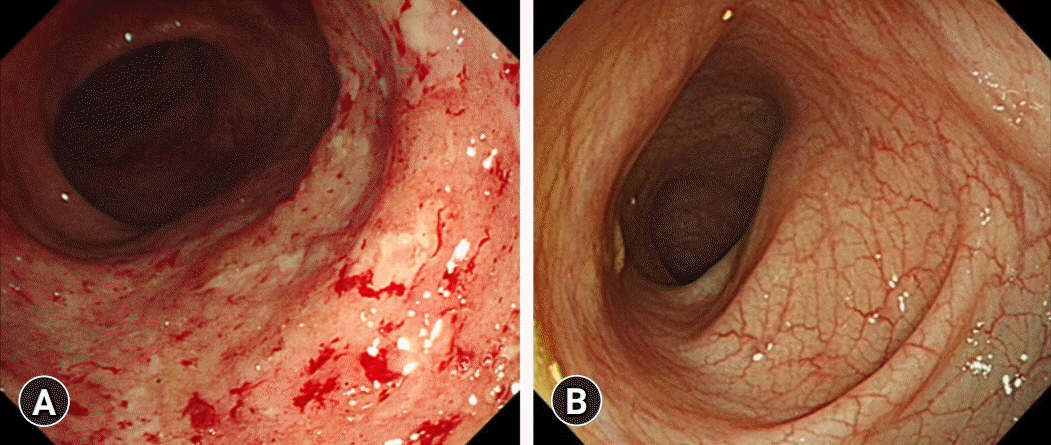
- Vedolizumab (VDZ) has been approved for the treatment of inflammatory bowel diseases (IBDs) in patients aged ≥18 years. We report a case of a pediatric patient with Crohn disease (CD) who was successfully treated with VDZ. A 16-year-old female developed severe active pulmonary tuberculosis (TB) during treatment with infliximab (IFX). IFX was stopped, and TB treatment was started. After a 6-month regimen of standard TB medication, her pulmonary TB was cured; however, gastrointestinal symptoms developed. Due to the concern of the patient and parents regarding TB reactivation on restarting treatment with IFX, VDZ was started off-label. After the second dose of VDZ, the patient was in clinical remission and her remission was continuously sustained. Ileocolonoscopy at 1-year after VDZ initiation revealed endoscopic healing. Therapeutic drug monitoring conducted during VDZ treatment showed negative antibodies to VDZ. No serious adverse events occurred during the VDZ treatment. This is the first case report in Korea demonstrating the safe and effective use of VDZ treatment in a pediatric CD patient. In cases that require recommencement of treatment with biologics after recovery of active pulmonary TB caused by anti-tumor necrosis factor agents, VDZ may be a good option even in pediatric IBD.
Figure
Cited by 1 articles
-
Vedolizumab Is Safe and Efficacious for the Treatment of Pediatric-Onset Inflammatory Bowel Disease Patients Who Fail a Primary Biologic Agent
Sujin Choi, Eun Sil Kim, Yiyoung Kwon, Mi Jin Kim, Yon Ho Choe, Byung-Ho Choe, Ben Kang
J Korean Med Sci. 2022;37(37):e282. doi: 10.3346/jkms.2022.37.e282.
Reference
-
References
1. Rosen MJ, Dhawan A, Saeed SA. Inflammatory bowel disease in children and adolescents. JAMA Pediatr. 2015; 169:1053–60.
Article2. Kang B, Choe YH. Early biologic treatment in pediatric Crohn’s disease: catching the therapeutic window of opportunity in early disease by treat-to-target. Pediatr Gastroenterol Hepatol Nutr. 2018; 21:1–11.
Article3. Kang B, Choi SY, Kim HS, Kim K, Lee YM, Choe YH. Mucosal healing in paediatric patients with moderate-to-severe luminal crohn's disease under combined immunosuppression: escalation versus early treatment. J Crohns Colitis. 2016; 10:1279–86.
Article4. Soler D, Chapman T, Yang LL, Wyant T, Egan R, Fedyk ER. The binding specificity and selective antagonism of vedolizumab, an anti-alpha4beta7 integrin therapeutic antibody in development for inflammatory bowel diseases. J Pharmacol Exp Ther. 2009; 330:864–75.
Article5. Feagan BG, Rutgeerts P, Sands BE, Hanauer S, Colombel JF, Sandborn WJ, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013; 369:699–710.
Article6. Sandborn WJ, Feagan BG, Rutgeerts P, Hanauer S, Colombel JF, Sands BE, et al. Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2013; 369:711–21.
Article7. Turner D, Ruemmele FM, Orlanski-Meyer E, Griffiths AM, de Carpi JM, Bronsky J, et al. Management of paediatric ulcerative colitis, part 1: ambulatory care-an evidence-based guideline from European Crohn’s and Colitis Organization and European Society of Paediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr. 2018; 67:257–91.
Article8. van Rheenen PF, Aloi M, Assa A, Bronsky J, Escher JC, Fagerberg UL, et al. The medical management of paediatric Crohn’s disease: an ECCO-ESPGHAN guideline update. J Crohns Colitis. 2021; 15:171–94.
Article9. Park DI, Hisamatsu T, Chen M, Ng SC, Ooi CJ, Wei SC, et al. Asian Organization for Crohn’s and Colitis and Asia Pacific Association of Gastroenterology consensus on tuberculosis infection in patients with inflammatory bowel disease receiving anti-tumor necrosis factor treatment. Part 1: risk assessment. Intest Res. 2018; 16:4–16.
Article10. Park DI, Hisamatsu T, Chen M, Ng SC, Ooi CJ, Wei SC, et al. Asian Organization for Crohn’s and Colitis and Asia Pacific Association of Gastroenterology consensus on tuberculosis infection in patients with inflammatory bowel disease receiving anti-tumor necrosis factor treatment. Part 2: management. Intest Res. 2018; 16:17–25.
Article11. Löwenberg M, Vermeire S, Mostafavi N, Hoentjen F, Franchimont D, Bossuyt P, et al. Vedolizumab induces endoscopic and histologic remission in patients with Crohn’s disease. Gastroenterology. 2019; 157:997–1006.
Article12. Colombel JF, Sands BE, Rutgeerts P, Sandborn W, Danese S, D’Haens G, et al. The safety of vedolizumab for ulcerative colitis and Crohn’s disease. Gut. 2017; 66:839–51.
Article13. Abreu C, Magro F, Santos-Antunes J, Pilão A, Rodrigues-Pinto E, Bernardes J, et al. Tuberculosis in anti-TNF-α treated patients remains a problem in countries with an intermediate incidence: analysis of 25 patients matched with a control population. J Crohns Colitis. 2013; 7:e486–92.
Article14. Ledder O, Assa A, Levine A, Escher JC, de Ridder L, Ruemmele F, et al. Vedolizumab in paediatric inflammatory bowel disease: a retrospective multi-centre experience from the paediatric IBD porto group of ESPGHAN. J Crohns Colitis. 2017; 11:1230–7.
Article15. Singh N, Rabizadeh S, Jossen J, Pittman N, Check M, Hashemi G, et al. Multi-center experience of vedolizumab effectiveness in pediatric inflammatory bowel disease. Inflamm Bowel Dis. 2016; 22:2121–6.
Article16. Sands BE, Feagan BG, Rutgeerts P, Colombel JF, Sandborn WJ, Sy R, et al. Effects of vedolizumab induction therapy for patients with Crohn’s disease in whom tumor necrosis factor antagonist treatment failed. Gastroenterology. 2014; 147:618–27.
Article17. Dulai PS, Boland BS, Singh S, Chaudrey K, Koliani-Pace JL, Kochhar G, et al. Development and validation of a scoring system to predict outcomes of vedolizumab treatment in patients with Crohn’s disease. Gastroenterology. 2018; 155:687–95.
Article18. Pouillon L, Vermeire S, Bossuyt P. Vedolizumab trough level monitoring in inflammatory bowel disease: a state-of-the-art overview. BMC Med. 2019; 17:89.
Article19. Dreesen E, Verstockt B, Bian S, de Bruyn M, Compernolle G, Tops S, et al. Evidence to support monitoring of vedolizumab trough concentrations in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2018; 16:1937–46.
Article20. Aardoom MA, Jongsma MM, de Vries A, Wolthoorn J, de Ridder L, Escher JC. Vedolizumab trough levels in children with anti-tumor necrosis factor refractory inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2020; 71:501–7.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Miliary Tuberculosis in a Patient with Behcet's Disease and Uveitis Receiving Infliximab
- Infliximab-Induced Lupus in Crohn's Disease
- The Risk of Tuberculosis in Patients With Inflammatory Bowel Disease Treated With Vedolizumab or Ustekinumab in Korea
- A Case of Multiple Tuberculosis Associated with Infliximab Therapy in Crohn's Disease
- Recent Trends of Infliximab Treatment for Crohn's Disease