Obstet Gynecol Sci.
2021 Jul;64(4):353-363. 10.5468/ogs.20316.
Vitamin D supplementation for primary dysmenorrhea: a double-blind, randomized, placebo-controlled trial
- Affiliations
-
- 1Department of Midwifery and Reproductive Health, School of Nursing and Midwifery, Midwifery and Reproductive Health Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 2Pain Management Research Institute, Faculty of Medicine and Health, The University of Sydney, Sydney, Australia
- 3Department of Obstetrics and Gynecology, Shohada Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 4Proteomics Research Center and Department of Biostatics, Faculty of Paramedical Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 5Department of Nutrition, School of Public Health, Iran University of Medical Sciences, Tehran, Iran
- 6Clinical Pathology Laboratory, Shohadae Tajrish Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- KMID: 2518378
- DOI: http://doi.org/10.5468/ogs.20316
Abstract
Objective
Recent studies have shown a possible association between vitamin D deficiency and the severity of primary dysmenorrhea. The present study aimed to investigate the effect of vitamin D supplementation on pain and systemic symptoms in patients with primary dysmenorrhea.
Methods
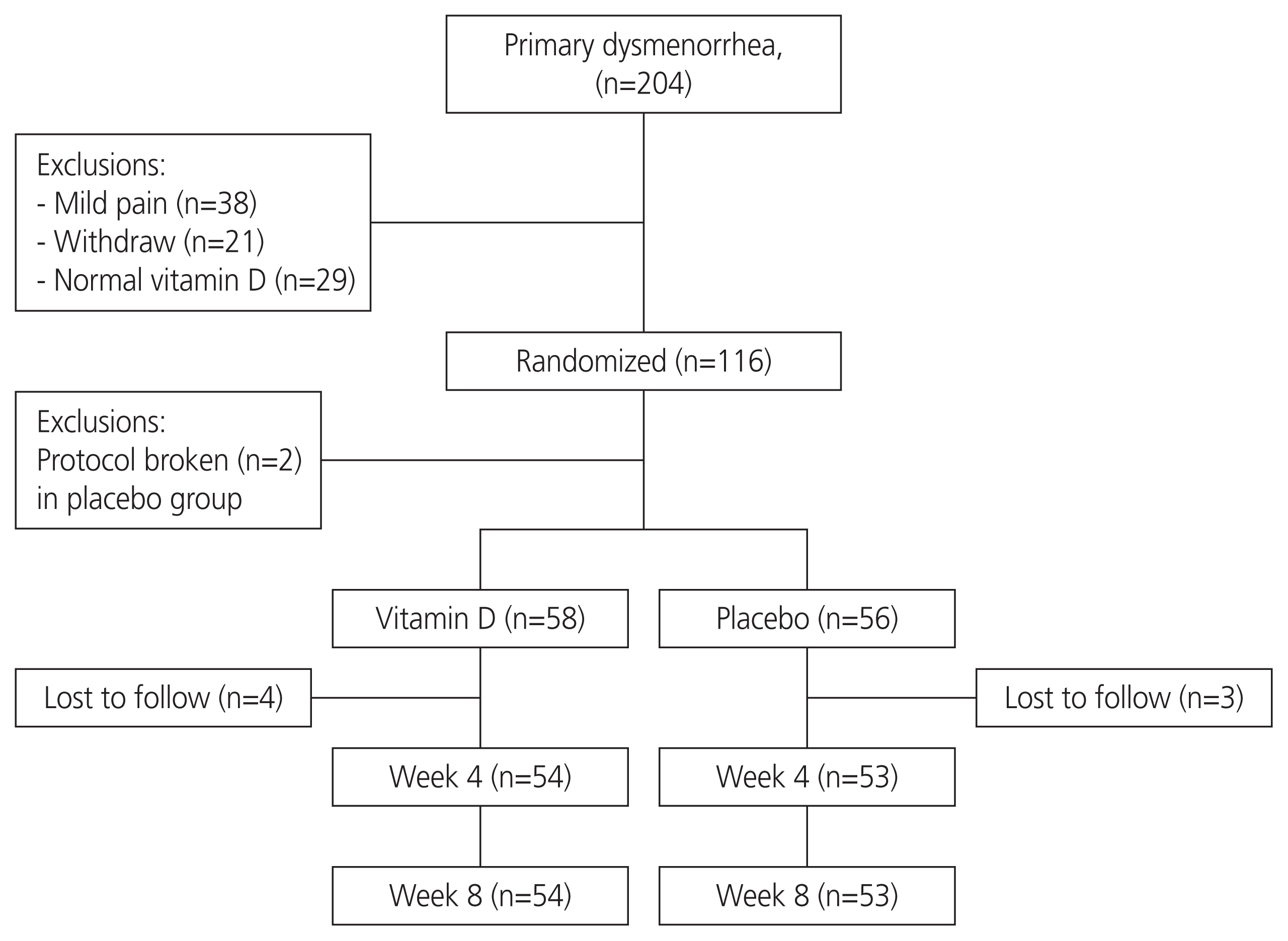
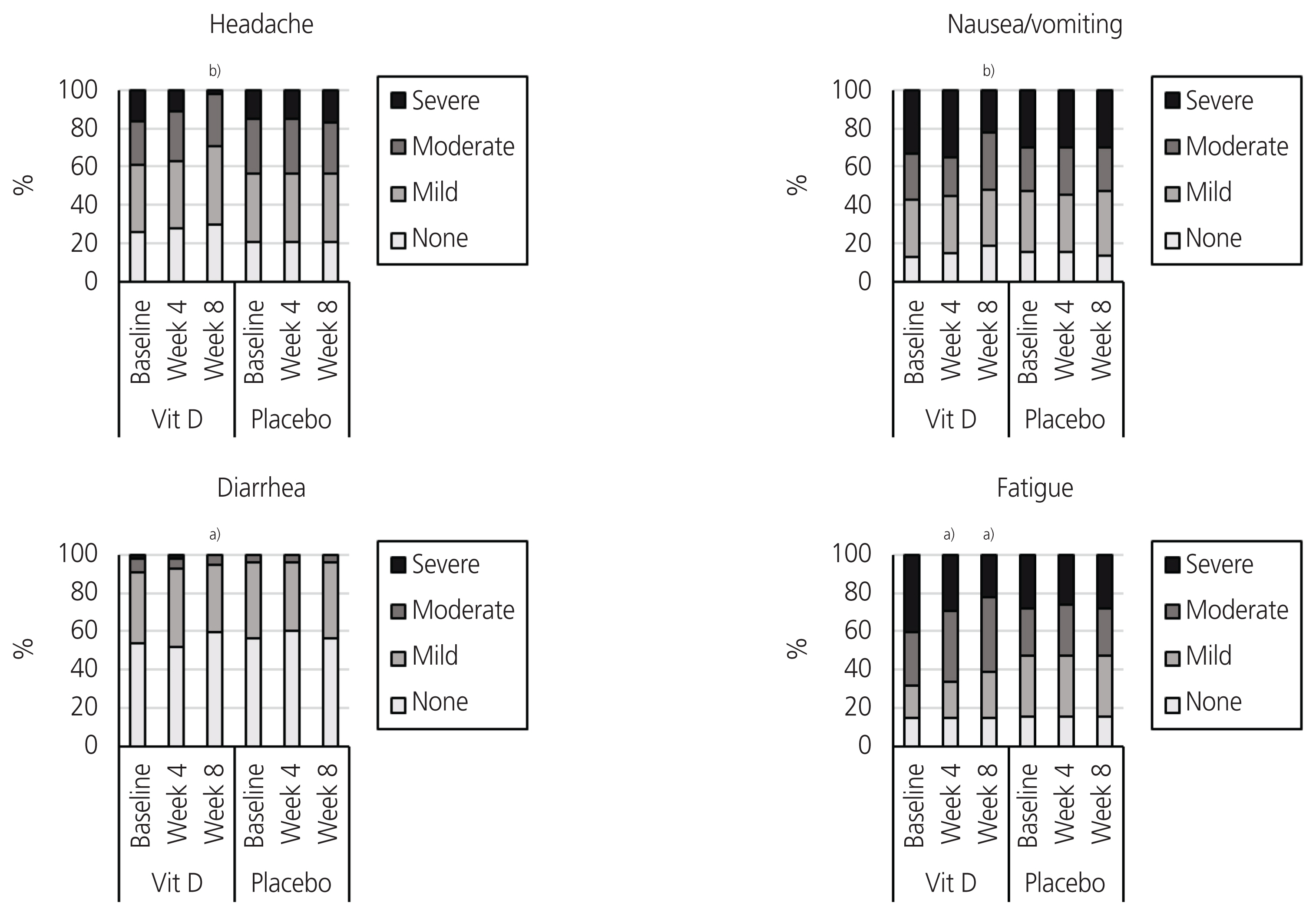
This double-blind, randomized, placebo-controlled trial was conducted on female students aged 18 to 32 years with primary dysmenorrhea and vitamin D deficiency (25 [OH]D <30 ng/mL). The participants (n=116) received either 50,000 IU of vitamin D3 (cholecalciferol) or placebo capsules on a weekly basis for eight consecutive weeks. The outcomes were pain intensity (scored 0 to 10), number of days with pain, number of consumed pain-relief medications (per day), and severity of systemic symptoms (fatigue, headache, nausea/vomiting, and diarrhea; total score of 0 to 12).
Results
Compared with baseline, our participants who received vitamin D experienced significant reductions in pain intensity (-1.0 and -1.5 score at weeks 4 and 8, P<0.001), the number of days with pain (-1.0 day at weeks 4 and 8, P<0.001), the number of consumed pain-relief medications (-1.0 at weeks 4 and 8, P<0.001), and systemic symptoms severity (-1.0 score at weeks 4 and 8, P<0.001). No significant improvements were observed in the placebo group in terms of these outcomes.
Conclusion
Vitamin D supplementation in women with primary dysmenorrhea and vitamin D deficiency could improve systemic symptoms and reduce pain intensity, the number of days with pain, and the need for consuming pain-relief medications.
Keyword
Figure
Cited by 1 articles
-
Effect of vitamin D vaginal suppository on sexual functioning among postmenopausal women: A three-arm randomized controlled clinical trial
Zinat Sarebani, Venus Chegini, Hui Chen, Ehsan Aali, Monirsadat Mirzadeh, Mohammadreza Abbaspour, Mark D. Griffiths, Zainab Alimoradi
Obstet Gynecol Sci. 2023;66(3):208-220. doi: 10.5468/ogs.22038.
Reference
-
References
1. ACOG Committee Opinion No. 760: Dysmenorrhea and endometriosis in the adolescent. Obstet Gynecol. 2018; 132:e249–58.2. Iacovides S, Avidon I, Baker FC. What we know about primary dysmenorrhea today: a critical review. Hum Reprod Update. 2015; 21:762–78.
Article3. Proctor M, Farquhar C. Diagnosis and management of dysmenorrhoea. BMJ. 2006; 332:1134–8.
Article4. Juang CM, Yen MS, Twu NF, Horng HC, Yu HC, Chen CY. Impact of pregnancy on primary dysmenorrhea. Int J Gynaecol Obstet. 2006; 92:221–7.
Article5. Ju H, Jones M, Mishra G. The prevalence and risk factors of dysmenorrhea. Epidemiol Rev. 2014; 36:104–13.
Article6. Kharaghani R, Damghanian M. The prevalence of dysmenorrhea in Iran: a systematic review and meta-analysis. Iran Red Crescent Med J. 2017; 19:e40856.
Article7. De Sanctis V, Soliman AT, Elsedfy H, Soliman NA, Elalaily R, El Kholy M. Dysmenorrhea in adolescents and young adults: a review in different countries. Acta Biomed. 2016; 87:233–46.8. Sultan C, Gaspari L, Paris F. Adolescent dysmenorrhea. Endocr Dev. 2012; 22:171–80.
Article9. Doty E, Attaran M. Managing primary dysmenorrhea. J Pediatr Adolesc Gynecol. 2006; 19:341–4.
Article10. Berek JS. Berek & Novak’s gynecology. 16th ed. Philadelphia (PA): Lippincott Williams & Wilkins;2019.11. Guimarães I, Póvoa AM. Primary dysmenorrhea: assessment and treatment. Rev Bras Ginecol Obstet. 2020; 42:501–7.
Article12. Zahradnik HP, Hanjalic-Beck A, Groth K. Nonsteroidal anti-inflammatory drugs and hormonal contraceptives for pain relief from dysmenorrhea: a review. Contraception. 2010; 81:185–96.
Article13. Firestein GS, Budd RC, Harris ED, MacInnes IB, Ruddy S, Sergent JS. Kelley´s textbook of rheumatology. 8th ed. Philadelphia (PA): Saunders Elsevier;2009.14. Pramanik P, Banerjee SB, Saha P. Primary dysmenorrhea in school going adolescent girls-is it related to deficiency of antioxidant in diet? Int J Life Sci Phar Res. 2015; 5:54–63.15. Charandabi SMA, Mirghafourvand M, Javadzadeh Y, Chegini SN. Effect of calcium with and without magnesium on amount and duration of menstrual bleeding in students with primary dysmenorrhea. Iran J Obestet Gynecol Infertil. 2013; 16:1–8.16. Abdi F, Ozgoli G, Rahnemaie FS. A systematic review of the role of vitamin D and calcium in premenstrual syndrome. Obstet Gynecol Sci. 2019; 62:73–86.
Article17. Abdi F, Amjadi MA, Zaheri F, Rahnemaei FA. Role of vitamin D and calcium in the relief of primary dysmenorrhea: a systematic review. Obstet Gynecol Sci. 2021; 64:13–26.
Article18. Thota C, Laknaur A, Farmer T, Ladson G, Al-Hendy A, Ismail N. Vitamin D regulates contractile profile in human uterine myometrial cells via NF-κB pathway. Am J Obstet Gynecol. 2014; 210:347.e1–10.
Article19. Viganò P, Lattuada D, Mangioni S, Ermellino L, Vignali M, Caporizzo E, et al. Cycling and early pregnant endometrium as a site of regulated expression of the vitamin D system. J Mol Endocrinol. 2006; 36:415–24.20. Kucukceran H, Ozdemir O, Kiral S, Berker DS, Kahveci R, Ozkara A, et al. The impact of circulating 25-hydroxyvitamin and oral cholecalciferol treatment on menstrual pain in dysmenorrheic patients. Gynecol Endocrinol. 2019; 35:53–7.
Article21. Lasco A, Catalano A, Benvenga S. Improvement of primary dysmenorrhea caused by a single oral dose of vitamin D: results of a randomized, double-blind, placebo-controlled study. Arch Intern Med. 2012; 172:366–7.
Article22. Zangene M, Veisi F, Nankali A, Rezaei M, Ataee M. Evaluation of the effects of oral vitamin-D for pelvic pain reduction in primary dysmenorrhea. Iran J Obstet Gynecol Infertil. 2014; 88:14–20.23. Moini A, Ebrahimi T, Shirzad N, Hosseini R, Radfar M, Bandarian F, et al. The effect of vitamin D on primary dysmenorrhea with vitamin D deficiency: a randomized double-blind controlled clinical trial. Gynecol Endocrinol. 2016; 32:502–5.
Article24. Ayşegül Ö, Seda A, Şevket O, Özdemir M, İlhan G, Davutoğlu E. A randomized controlled study of vitamin D in the treatment of primary dysmenorrhea. Duzce Med J. 2019; 21:32–6.25. Vatandost S, Jahani M, Afshari A, Amiri MR, Heidarimoghadam R, Mohammadi Y. Prevalence of vitamin D deficiency in Iran: a systematic review and meta-analysis. Nutr Health. 2018; 24:269–78.
Article26. Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011; 96:1911–30.
Article27. Beller EM, Gebski V, Keech AC. Randomisation in clinical trials. Med J Aust. 2002; 177:565–7.
Article28. Hodges JL Jr, Lehmann EL. Estimates of location based on rank tests. Ann Math Stat. 1963; 34:598–611.
Article29. Holm S. A simple sequentially rejective multiple test procedure. Scand J Statist. 1979; 6:65–70.30. Abdul-Razzak KK, Ayoub NM, Abu-Taleb AA, Obeidat BA. Influence of dietary intake of dairy products on dysmenorrhea. J Obstet Gynaecol Res. 2010; 36:377–83.
Article31. Abdul-Razzak KK, Obeidat BA, Al-Farras MI, Dauod AS. Vitamin D and PTH status among adolescent and young females with severe dysmenorrhea. J Pediatr Adolesc Gynecol. 2014; 27:78–82.
Article32. Holick MF. Vitamin D deficiency. N Engl J Med. 2007; 357:266–81.
Article33. Adams JS, Hewison M. Extrarenal expression of the 25-hydroxyvitamin D-1-hydroxylase. Arch Biochem Biophys. 2012; 523:95–102.
Article34. Abbasi ST, Abbasi P, Suhag AH, Qureshi MA. Serum magnesium and 25-hydroxy cholecalciferol in premenstrual syndrome during luteal phase. J Liaquat Uni Med Health Sci. 2017; 16:209–12.
Article35. Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Guidelines for preventing and treating vitamin D deficiency and insufficiency revisited. J Clin Endocrinol Metab. 2012; 97:1153–8.
Article36. Fassio A, Adami G, Rossini M, Giollo A, Caimmi C, Bixio R, et al. Pharmacokinetics of oral cholecalciferol in healthy subjects with vitamin d deficiency: a randomized open-label study. Nutrients. 2020; 12:1553.
Article37. Toth BE, Takacs I, Szekeres L, Szabo B, Bakos B. Safety and efficacy of weekly 30,000 IU vitamin D supplementation as a slower loading dose administration compared to a daily maintenance schedule in deficient patients: a randomized. Contr Clin Trial J Pharmacovigil. 2017; 5:2.38. Kearns MD, Alvarez JA, Tangpricha V. Large, single-dose, oral vitamin D supplementation in adult populations: a systematic review. Endocr Pract. 2014; 20:341–51.
Article39. Karacin O, Mutlu I, Kose M, Celik F, Kanat-Pektas M, Yilmazer M. Serum vitamin D concentrations in young Turkish women with primary dysmenorrhea: a randomized controlled study. Taiwan J Obstet Gynecol. 2018; 57:58–63.
Article40. Thys-Jacobs S, McMahon D, Bilezikian JP. Cyclical changes in calcium metabolism across the menstrual cycle in women with premenstrual dysphoric disorder. J Clin Endocrinol Metab. 2007; 92:2952–9.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of multivitamin on serum 25-hydroxy vitamin D level in postmenopausal women: A randomized, double-blind, placebo-controlled trial
- Comparison of the effect of vitamin E, vitamin D and ginger on the severity of primary dysmenorrhea: a single-blind clinical trial
- Probiotic Supplementation for Treatment of Helicobacter pylori Infection: A Double-Blind Randomized Clinical Trial
- Effect of onion peel extract supplementation on the lipid profile and antioxidative status of healthy young women: a randomized, placebo-controlled, double-blind, crossover trial
- Occurrence of infections in schoolchildren subsequent to supplementation with vitamin D-calcium or zinc: a randomized, double-blind, placebo-controlled trial