J Korean Med Sci.
2021 Jul;36(27):e196. 10.3346/jkms.2021.36.e196.
Emergency Department Utilization by In-hospital Healthcare Workers after COVID-19 Vaccination
- Affiliations
-
- 1Department of Emergency Medicine, Ajou University School of Medicine, Suwon, Korea
- KMID: 2518354
- DOI: http://doi.org/10.3346/jkms.2021.36.e196
Abstract
- Background
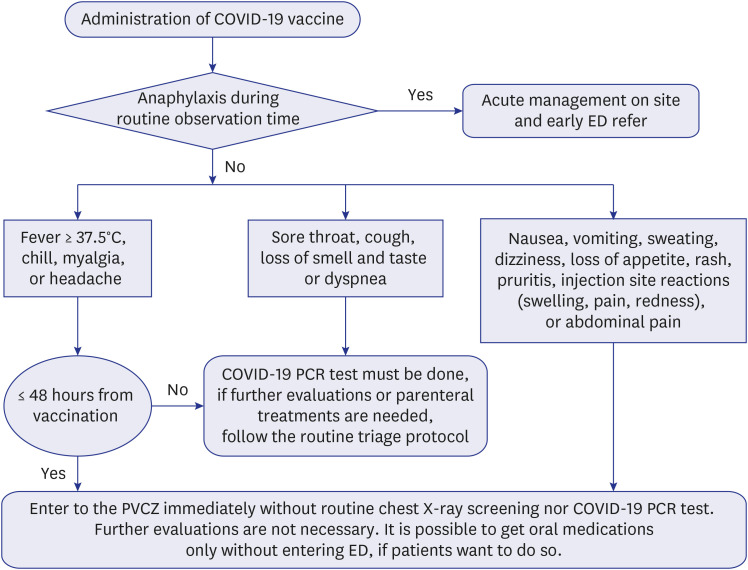
This is an observational study to analyze an emergency department (ED) utilization pattern of coronavirus disease 2019 (COVID-19) vaccinated in-hospital healthcare workers (HCWs).
Methods
We included 4,703 HCWs who were administered the first dose of the COVID-19 vaccine between March 4 and April 2, 2021, in a tertiary hospital in Korea where fast-track and post-vaccination cohort zone (PVCZ) were introduced in ED. We analyzed data of participants' age, sex, occupation, date and type of vaccination, and their clinical information using SPSS v25.0.
Results
The sample comprised HCWs, who received either the ChAdOx1 (n = 4,458) or the BNT162B2 (n = 245) vaccines; most participants were female (73.5%), and 81.1% were under 50 years old. Further, 153 (3.3%) visited the ED and reported experiencing fever (66.9%) and myalgia (56.1%). Additionally, 91(59.5%) of them were in their 20s, and 106 (67.5%) were assigned to the PVCZ. Lastly, 107 (68.2%) of the patients received parenteral management. No patient required hospitalization.
Conclusion
In conclusion, vaccinated HCWs who visited the ED with adverse events had a high incidence of fever and a low likelihood of developing serious illnesses. As the COVID-19 vaccination program for Korean citizens continues to expand, strategies to minimize unnecessary ED overcrowding should be put into effect.
Keyword
Figure
Reference
-
1. World Health Organization. WHO coronavirus disease (COVID-19) dashboard. Updated 2021. Accessed June 1, 2021. https://covid19.who.int.2. McKee M, Stuckler D; Martin MaKee. If the world fails to protect the economy, COVID-19 will damage health not just now but also in the future. Nat Med. 2020; 26(5):640–642. PMID: 32273610.
Article3. Korea Disease Control and Prevention Agency. Accessed June 2, 2021. https://ncv.kdca.go.kr/menu.es?mid=a10117010000.4. Bae S, Lee YW, Lim SY, Lee JH, Lim JS, Lee S, et al. Adverse reactions following the first dose of ChAdOx1 nCoV-19 Vaccine and BNT162b2Vaccine for healthcare workers in South Korea. J Korean Med Sci. 2021; 36(17):e115. PMID: 33942579.
Article5. Tobaiqy M, Elkout H, MacLure K. Analysis of thrombotic adverse reactions of COVID-19 Astrazeneca vaccine reported to EudraVigilance database. Vaccines (Basel). 2021; 9(4):393. PMID: 33923530.
Article6. Jeon M, Kim J, Oh CE, Lee JY. Adverse events following Immunization associated with coronavirus disease 2019 vaccination reported in the mobile vaccine adverse events reporting system. J Korean Med Sci. 2021; 36(17):e114. PMID: 33942578.
Article7. Kim SH, Wi YM, Yun SY, Ryu JS, Shin JM, Lee EH, et al. Adverse events in healthcare workers after the first dose of ChAdOx1 nCoV-19 or BNT162b2 mRNA COVID-19 vaccination: a single center experience. J Korean Med Sci. 2021; 36(14):e107. PMID: 33847085.
Article8. Fertel BS, Milk J, Simon EL, Muir MR, Smalley CM. COVID-19 vaccine adverse reactions bring patients to emergency departments. Am J Emerg Med. Forthcoming. 2021; DOI: 10.1016/j.ajem.2021.05.013.
Article9. Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021; 397(10269):99–111. PMID: 33306989.10. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020; 383(27):2603–2615. PMID: 33301246.
Article11. Ramasamy MN, Minassian AM, Ewer KJ, Flaxman AL, Folegatti PM, Owens DR, et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2021; 396(10267):1979–1993. PMID: 33220855.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Enhancing COVID-19 vaccination coverage using financial incentives: arguments to help health providers counterbalance erroneous claims
- Burnout among Healthcare Workers during COVID-19 Pandemic
- Factors Influencing COVID-19 AstraZeneca (ChAdOx1) Vaccination and Side Effects among Health Care Workers in an Acute General Hospital
- Factors Affecting Fear of COVID-19 Infection in Healthcare Workers in COVID-19 Dedicated Teams: Focus on Professional Quality of Life
- A Case of Aphthous Stomatitis in a Healthy Adult Following COVID-19 Vaccination: Clinical Reasoning