Kosin Med J.
2021 Jun;36(1):1-13. 10.7180/kmj.2021.36.1.1.
Antinociceptive Effect of BPC-157 in the Formalin-induced Pain Model
- Affiliations
-
- 1Department of Anesthesiology and Pain Medicine, Dong-A University College of Medicine, Busan, Korea
- KMID: 2517221
- DOI: http://doi.org/10.7180/kmj.2021.36.1.1
Abstract
Objectives
Body protective compound-157 (BPC-157) is a stable gastric pentadecapeptide that has been effective in trials aiming to increase wound healing capabilities and decrease inflammatory cell influx, including studies on the healing of muscles and tendons. There are no studies about the effect of BPC-157 on pain transmission via nociception. This study examined the antinociceptive effects of BPC-157 using formalin tests and immunohistochemistry.
Methods
Rats were randomly divided into the control, morphine and BPC-157 groups. Pain behavior was quantified periodically at 5- and 35- min intervals (representative values of phases 1 and 2) by counting the number of flinches exhibited by the injected paw after injection. The dorsal root ganglia (DRG) and spinal cords (SC) were collected, and then, the number of cytokine-positive cells was determined via immunostaining.
Results
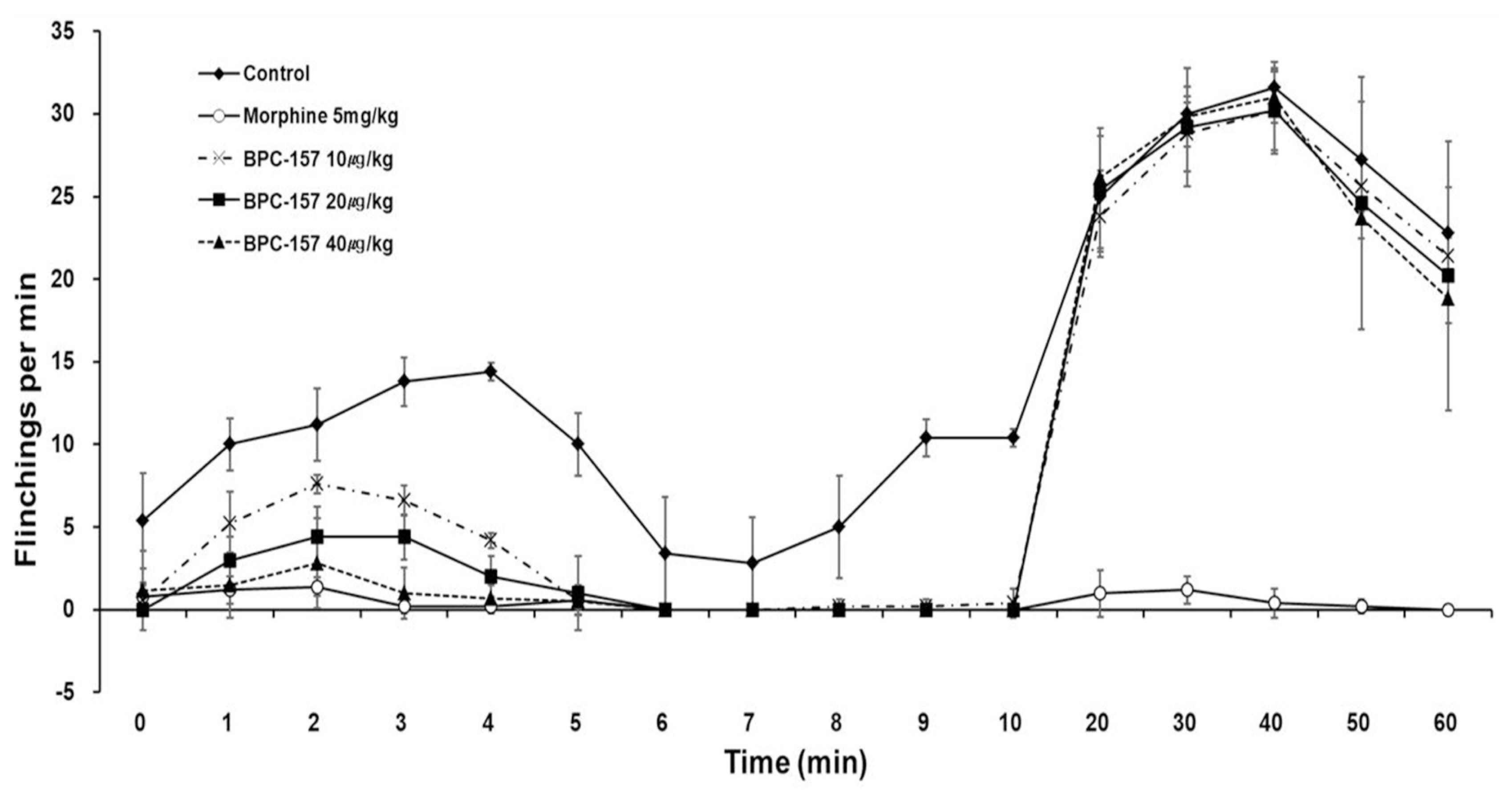
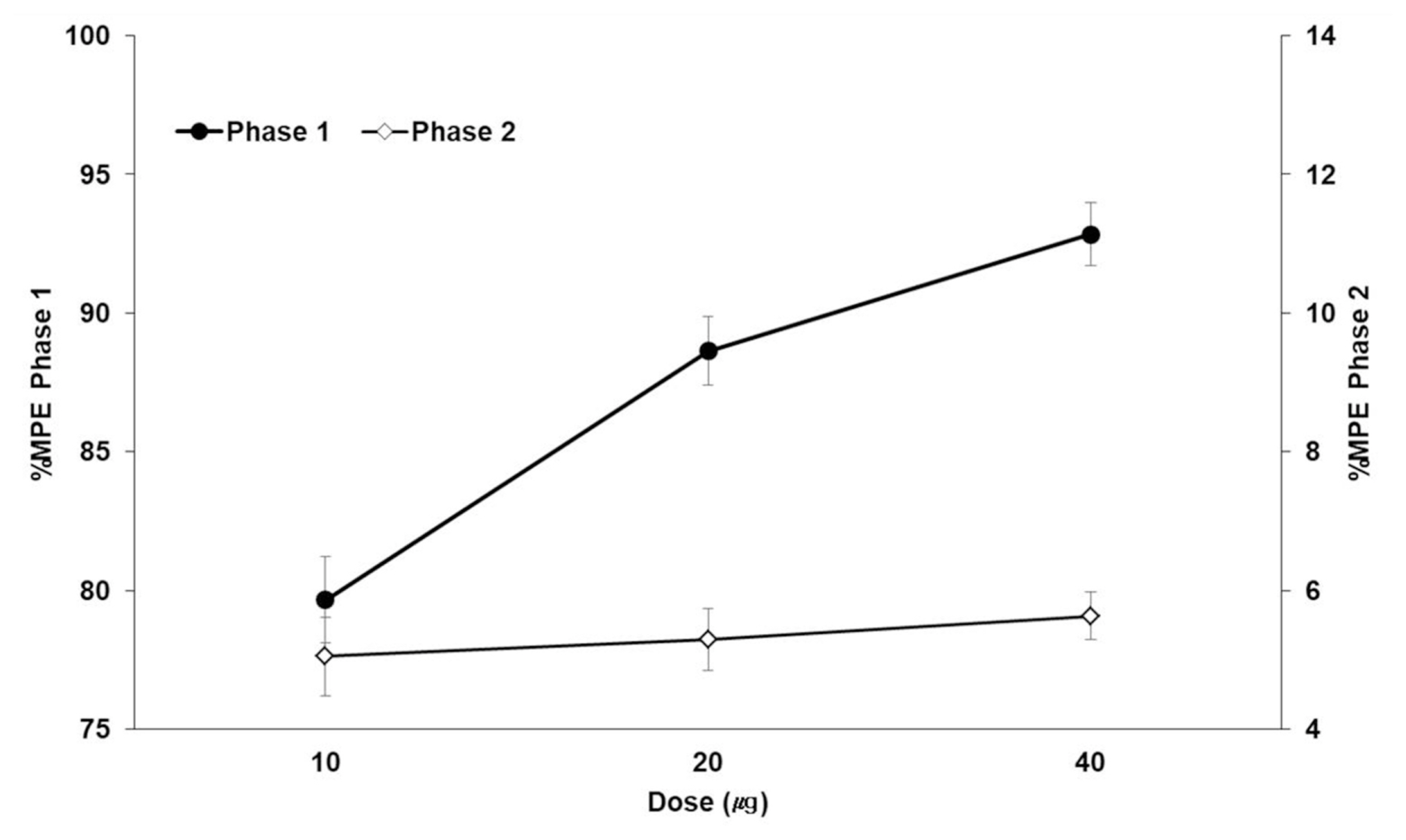
BPC-157 dose-dependently decreased the number of flinches during phase 1 but did not decrease the number of flinches during phase 2. During phase 1, interleukin-1β (IL-1β) in the DRG tissue was significantly different in the morphine, 10 μg/kg BPC-157, and 20 μg/kg BPC-157 groups. During phase 2, statistical significance was achieved in the DRG tissue in the morphine, 20 μg/kg BPC-157, and 40 μg/kg BPC-157 groups. During phase 1, interleukin-6 was significantly different in the DRG tissue in the morphine group and the SC tissue in the 10 μg/kg BPC-157 group. During phase 2, statistical significance was achieved in the morphine group and the BPC-157 20 μg/kg group in both the DRG and SC tissues. There were no significant differences in tumor necrosis factor-α between the DRG and SC tissues.
Conclusions
BPC-157 was effective during phase 1 but not during phase 2, as determined by the formalin test. BPC-157 decreased the expression of IL-1β in the DRG tissue in phases 1 and 2.
Keyword
Figure
Reference
-
1. Sikiric P, Seiwerth S, Rucman R, Kolenc D, Vuletic LB, Drimic D, et al. Brain-gut axis and pentadecapeptide BPC 157: theoretical and practical implications. Curr Neuropharmacol. 2016; 14:857–65.
Article2. Sawynok J, Liu XJ. The formalin test: characteristics and usefulness of the model. Rev Analg. 2003; 7:145–63.
Article3. Dickenson AH, Sullivan AF. Subcutaneous formalin-induced activity of dorsal horn neurones in the rat: differential response to an intrathecal opiate administered pre or post formalin. Pain. 1987; 30:349–60.
Article4. Coderre TJ, Vaccarino AL, Melzack R. Central nervous system plasticity in the tonic pain response to subcutaneous formalin injection. Brain Res. 1990; 535:155–8.
Article5. Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983; 16:109–10.
Article6. Sevostianova N, Zvartau E, Bespalov A, Danysz W. Effects of morphine on formalin-induced nociception in rats. Eur J Pharmacol. 2003; 462:109–13.
Article7. Dubuisson D, Dennis SG. The formalin test: A quantitative study of the analgesic effects of morphine, meperidine, and brain stem stimulation in rats and cats. Pain. 1977; 4:161–74.
Article8. Tatsuo Y, Natsuko NT, Tanemichi C. Analgesic effect of intrathecally administered orexin-A in the rat formalin test and in the rat hot plate test. Br J Pharmacol. 2002; 137:170–6.9. McCall WD, Tanner KD, Levine JD. Formalin induces biphasic activity in C-fibers in the rat. Neurosci Lett. 1996; 208:45–8.
Article10. Yaksh TL, Hua XY, Kalcheva I, Nozaki-Taguchi N, Marsala M. The spinal biology in humans and animals of pain states generated by persistent small afferent input. Proc Natl Acad Sci USA. 1999; 96:7680–6.
Article11. Moalem G, Tracey DJ. Immune and inflammatory mechanisms in neuropathic pain. Brain Res Rev. 2006; 51:240–64.
Article12. Woolf CJ, Allchorne A, Safieh-Garabedian B, Poole S. Cytokines, nerve growth factor and inflammatory hyperalgesia: The contribution of tumour necrosis factor α. Br J Pharmacol. 1997; 121:417–24.
Article13. Dinarello CA. Interleukin-1. Cytokine Growth Factor Rev. 1997; 8:253–65.
Article14. Zhou YQ, Liu Z, Liu ZH, Chen SP, Li M, Shahveranov A, et al. Interleukin-6: an emerging regulator of pathological pain. J Neuroinflammation. 2016; 13:141.
Article15. Wiltse LL. Anatomy of the extradural compartments of the lumbar spinal canal. Peridural membrane and circumneural sheath. Radiol Clin North Am. 2000; 38:1177–206.16. Abram SE, Yi J, Fuchs A, Hogan QH. Permeability of injured and intact peripheral nerves and dorsal root ganglia. Anesthesiology. 2006; 105:146–53.
Article17. Kiernan JA, Rajakumar N. Barr’s The Human Nervous System: An Anatomical Viewpoint. 10th ed. Lippincott Williams & Wilkins Publishs;2013.18. Gjurasin M, Miklic P, Zupancic B, Perovic D, Zarkovic K, Brcic L, et al. Peptide therapy with pentadecapeptide BPC 157 in traumatic nerve injury. Regul Pept. 2010; 160:33–41.
Article19. Boban Blagaic A, Turcic P, Blagaic V, Dubovecak M, Jelovac N, Zemba M, et al. Gastric pentadecapeptide BPC 157 counter-acts morphine-induced analgesia in mice. J Physiol Pharmacol. 2009; 60:177–81.20. Haley JE, Dickenson AH. Evidence for spinal N-methyl-D-aspartate receptor involvement in prolonged chemical nociception in the rat. Brain Res. 2016; 1645:58–60.
Article21. Rivot JP, Montagne-Clavel J, Besson JM. Subcutaneous formalin and intraplantar carrageenan increase nitric oxide release as measured by in vivo voltammetry in the spinal cord. Eur J Pain. 2002; 6:25–34.
Article22. Terenghi G, Riveros-Moreno V, Hudson LD, Ibrahim NB, Polak JM. Immunohistochemistry of nitric oxide synthase demonstrates immunoreactive neurons in spinal cord and dorsal root ganglia of man and rat. J Neurol Sci. 1993; 118:34–7.
Article23. Haley JE, Dickenson AH, Schachter M. Electrophysiological evidence for a role of nitric oxide in prolonged chemical nociception in the rat. Neuropharmacology. 1992; 31:251–8.
Article24. Sikiric P, Seiwerth S, Rucman R, Turkovic B, Rokotov DS, Brcic L, et al. Stable gastric pentadecapeptide BPC 157-NO-system relation. Curr Pharm Des. 2014; 20:1126–35.
Article25. Sikirić P, Petek M, Rucman R, Seiwerth S, Grabarević Z, Rotkvić I, et al. A new gastric juice peptide, BPC. An overview of the stomach-stress-organoprotection hypothesis and beneficial effects of BPC. J Physiol Paris. 1993; 87:313–27.
Article26. Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991; 43:109–42.27. Veljaca M, Lesch CA, Pllana R, Sanchez B, Chan K, Guglietta A. BPC-15 reduces trinitrobenzene sulfonic acid-induced colonic damage in rats. J Pharmacol Exp Ther. 1995; 272:417–22.28. Sikirić P, Seiwerth S, Grabarević Z, Rucman R, Petek M, Jagić V, et al. The influence of a novel pentadecapeptide, BPC 157, on N(G)-nitro-L-arginine methylester and L-arginine effects on stomach mucosa integrity and blood pressure. Eur J Pharmacol. 1997; 3(32):23–33.
Article29. Smullin DH, Skilling SR, Larson AA. Interactions between substance P, calcitonin gene-related peptide, taurine and excitatory amino acids in the spinal cord. Pain. 1990; 42:93–101.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The anti-nociceptive effect of BPC-157 on the incisional pain model in rats
- Antinociceptive Effect of Intrathecal Nefopam and Interaction with Morphine in Formalin-Induced Pain of Rats
- The Antinociceptive Effect of Intraperitoneally Administered Nonselective Nitric Oxide Synthase Inhibitor on the Rat Formalin Test
- Anti-inflammatory and antinociceptive effects of sitagliptin in animal models and possible mechanisms involved in the antinociceptive activity
- Spinal Noradrenergic Modulation and the Role of the Alpha-2 Receptor in the Antinociceptive Effect of Intrathecal Nefopam in the Formalin Test