Anat Cell Biol.
2021 Jun;54(2):249-258. 10.5115/acb.20.320.
Metformin treatment confers protection of the optic nerve following photoreceptor degeneration
- Affiliations
-
- 1Department of Histology and Cell Biology, Faculty of Medicine, Assiut University, Assiut, Egypt
- KMID: 2516909
- DOI: http://doi.org/10.5115/acb.20.320
Abstract
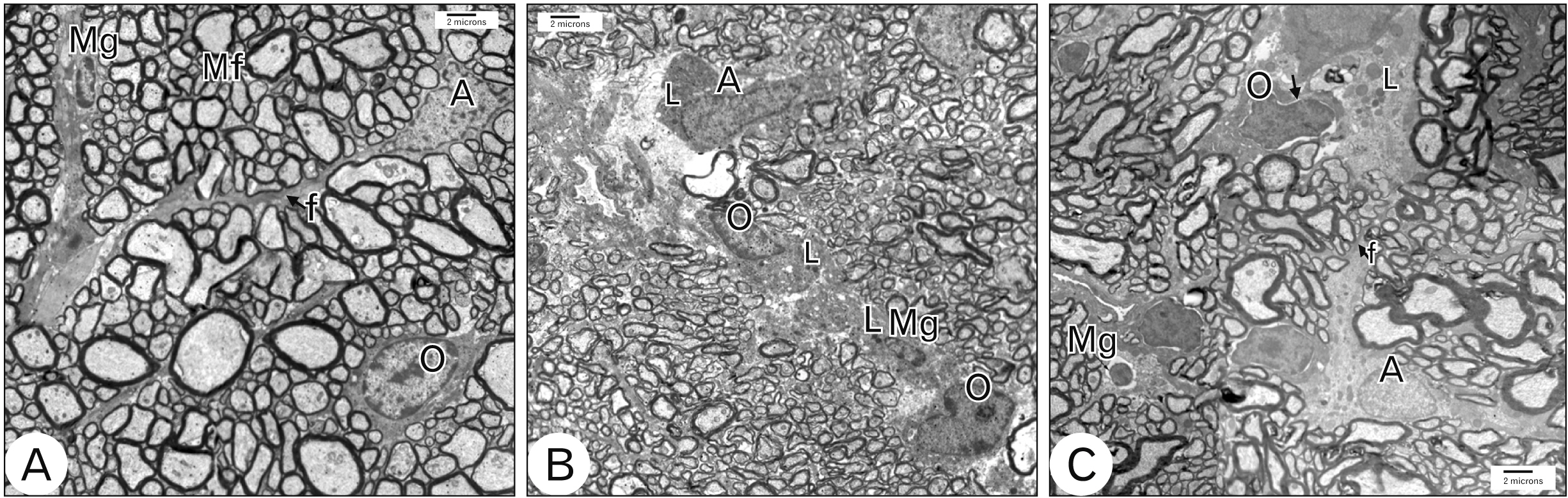
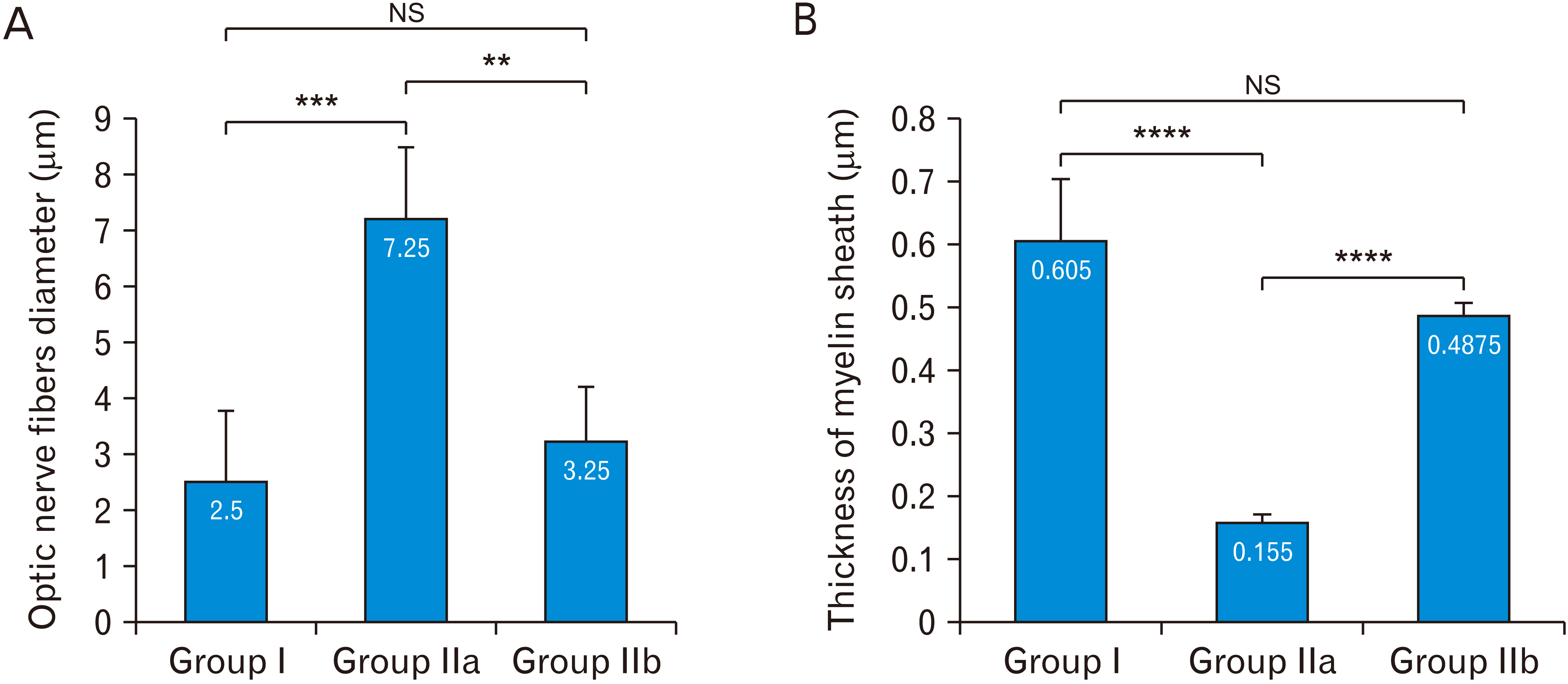
- Acquired or inherited or photoreceptor loss causes retinal ganglion cell loss and ultimately axonal transport alteration. Thus, therapies should be applied early during photoreceptors degeneration before the remodeling process reaches the inner retina. This study aimed to evaluate the protective effect of metformin on the rat optic nerve following photoreceptors loss induced by N-Ethyl-N-nitrosourea (ENU). Eighteen adults male Wistar rats were divided into two groups. Group I: normal vehicle control (n=6). Group II: ENU-induced photoreceptors degeneration (n=12) received a single intraperitoneal injection of ENU at a dose of 600 mg/kg. Rats in group II were equally divided into two subgroups: IIa: photoreceptor degeneration induced group and IIb: metformin treated group (200 mg/kg) for 7 days. Specimens from the optic nerve were processed for light and electron microscopy. In ENU treated group, the optic nerve revealed reduction in the diameter of the optic nerve fibers and thinning of myelin sheath with morphological changes in the glia (astrocytes, oligodendrocytes, and microglia). Caspase-3 (apoptotic marker), iNOS (oxidative stress marker) and CD68 (macrophage marker) expression increased. In metformin-treated group, the diameter of optic nerve fibers and myelin sheath thickness increased with improvement of the deterioration in the glia. Caspase-3, iNOS and CD68 expression decreased. Metformin ameliorates the histological changes of the rat optic nerve following photoreceptors loss induced by ENU.
Figure
Cited by 1 articles
-
The role of hesperidin in ameliorating retinal changes in rats with experimentally induced type 1 diabetes mellitus and the active role of vascular endothelial growth factor and glial fibrillary acidic protein
Azza Saad Shehata, Dalia Abduelmoein Mohamed, Sherein Mahmoud Hagras, Shimaa Mohsen El-Beah, Heba Mohamed Elnegris
Anat Cell Biol. 2021;54(4):465-478. doi: 10.5115/acb.21.105.
Reference
-
References
1. García-Ayuso D, Di Pierdomenico J, Vidal-Sanz M, Villegas-Pérez MP. 2019; Retinal ganglion cell death as a late remodeling effect of photoreceptor degeneration. Int J Mol Sci. 20:4649. DOI: 10.3390/ijms20184649. PMID: 31546829. PMCID: PMC6770703.
Article2. Maaswinkel H, Mason B, Li L. 2003; ENU-induced late-onset night blindness associated with rod photoreceptor cell degeneration in zebrafish. Mech Ageing Dev. 124:1065–71. DOI: 10.1016/j.mad.2003.08.004. PMID: 14659595.
Article3. Marco-Gomariz MA, Hurtado-Montalbán N, Vidal-Sanz M, Lund RD, Villegas-Pérez MP. 2006; Phototoxic-induced photoreceptor degeneration causes retinal ganglion cell degeneration in pigmented rats. J Comp Neurol. 498:163–79. DOI: 10.1002/cne.21028. PMID: 16856141.
Article4. Garcia-Ayuso D, Di Pierdomenico J, Agudo-Barriuso M, Vidal-Sanz M, Villegas-Pérez MP. 2018; Retinal remodeling following photoreceptor degeneration causes retinal ganglion cell death. Neural Regen Res. 13:1885–6. DOI: 10.4103/1673-5374.239436. PMID: 30233058. PMCID: PMC6183041.
Article5. Qi B, Hu L, Zhu L, Shang L, Sheng L, Wang X, Liu N, Wen N, Yu X, Wang Q, Yang Y. 2017; Metformin attenuates cognitive impairments in hypoxia-ischemia neonatal rats via improving remyelination. Cell Mol Neurobiol. 37:1269–78. DOI: 10.1007/s10571-016-0459-8. PMID: 28035478.
Article6. Oda SS. 2017; Metformin protects against experimental acrylamide neuropathy in rats. Drug Dev Res. 78:349–59. DOI: 10.1002/ddr.21400. PMID: 28771761.
Article7. Yoshizawa K, Sasaki T, Uehara N, Kuro M, Kimura A, Kinoshita Y, Miki H, Yuri T, Tsubura A. 2012; N-ethyl-N-nitrosourea induces retinal photoreceptor damage in adult rats. J Toxicol Pathol. 25:27–35. DOI: 10.1293/tox.25.27. PMID: 22481856. PMCID: PMC3320154.8. Mao-Ying QL, Kavelaars A, Krukowski K, Huo XJ, Zhou W, Price TJ, Cleeland C, Heijnen CJ. 2014; The anti-diabetic drug metformin protects against chemotherapy-induced peripheral neuropathy in a mouse model. PLoS One. 9:e100701. DOI: 10.1371/journal.pone.0100701. PMID: 24955774. PMCID: PMC4067328.
Article9. Zhao RR, Xu XC, Xu F, Zhang WL, Zhang WL, Liu LM, Wang WP. 2014; Metformin protects against seizures, learning and memory impairments and oxidative damage induced by pentylenetetrazole-induced kindling in mice. Biochem Biophys Res Commun. 448:414–7. DOI: 10.1016/j.bbrc.2014.04.130. PMID: 24802403.
Article10. Drury RA, Wallington EA. 1980. Carleton's histological technique. 5th ed. Oxford University Press;Oxford: p. 15–21.11. Gupta PD. 1983; Ultrastructural study on semithin section. Sci Tools. 30:6–7.12. Yoshizawa K, Kuro-Kuwata M, Sasaki T, Lai YC, Kanematsu S, Miki H, Kimura-Kawanaka A, Uehara N, Yuri T, Tsubura A. 2011; Retinal degeneration induced in adult mice by a single intraperitoneal injection of N-ethyl-N-nitrosourea. Toxicol Pathol. 39:606–13. DOI: 10.1177/0192623311402221. PMID: 21498792.
Article13. Garcia-Martin E, Pinilla I, Sancho E, Almarcegui C, Dolz I, Rodriguez-Mena D, Fuertes I, Cuenca N. 2012; Optical coherence tomography in retinitis pigmentosa: reproducibility and capacity to detect macular and retinal nerve fiber layer thickness alterations. Retina. 32:1581–91. DOI: 10.1097/IAE.0b013e318242b838. PMID: 22922847.14. Oishi A, Ogino K, Nakagawa S, Makiyama Y, Kurimoto M, Otani A, Yoshimura N. 2013; Longitudinal analysis of the peripapillary retinal nerve fiber layer thinning in patients with retinitis pigmentosa. Eye (Lond). 27:597–604. DOI: 10.1038/eye.2013.34. PMID: 23519274. PMCID: PMC3650280.
Article15. Zhang Q. 2016; Retinitis pigmentosa: progress and perspective. Asia Pac J Ophthalmol (Phila). 5:265–71. DOI: 10.1097/APO.0000000000000227. PMID: 27488069.16. Lahav M, Craft J, Albert DM, Ishii Y. 1982; Advanced pigmentary retinal degeneration: an ultrastructural study. Retina. 2:65–75. DOI: 10.1097/00006982-198200220-00002. PMID: 7178679.17. Asakawa K, Ishikawa H, Uga S, Mashimo K, Kondo M, Terasaki H. 2016; Histopathological changes of inner retina, optic disc, and optic nerve in rabbit with advanced retinitis pigmentosa. Neuroophthalmology. 40:286–91. DOI: 10.1080/01658107.2016.1229339. PMID: 27928420. PMCID: PMC5120756.
Article18. A L, Zou T, He J, Chen X, Sun D, Fan X, Xu H. 2019; Rescue of retinal degeneration in rd1 mice by intravitreally injected metformin. Front Mol Neurosci. 12:102. DOI: 10.3389/fnmol.2019.00102. PMID: 31080404. PMCID: PMC6497809.
Article19. Goldenberg-Cohen N, Guo Y, Margolis F, Cohen Y, Miller NR, Bernstein SL. 2005; Oligodendrocyte dysfunction after induction of experimental anterior optic nerve ischemia. Invest Ophthalmol Vis Sci. 46:2716–25. DOI: 10.1167/iovs.04-0547. PMID: 16043843.
Article20. Renner M, Stute G, Alzureiqi M, Reinhard J, Wiemann S, Schmid H, Faissner A, Dick HB, Joachim SC. 2017; Optic nerve degeneration after retinal ischemia/reperfusion in a rodent model. Front Cell Neurosci. 11:254. DOI: 10.3389/fncel.2017.00254. PMID: 28878627. PMCID: PMC5572359.
Article21. Houshmand F, Barati M, Golab F, Ramezani-Sefidar S, Tanbakooie S, Tabatabaei M, Amiri M, Sanadgol N. 2019; Metformin-induced AMPK activation stimulates remyelination through induction of neurotrophic factors, downregulation of NogoA and recruitment of Olig2+ precursor cells in the cuprizone murine model of multiple sclerosis. Daru. 27:583–92. DOI: 10.1007/s40199-019-00286-z. PMID: 31620963. PMCID: PMC6895294.
Article22. Paintlia A, Paintlia M, Mohan S, Singh A, Singh I. 2013; Anti-diabetic drug, metformin protects oligodendrocytes under CNS pathological conditions: implication for multiple sclerosis (P5205). J Immunol. 190(1 Supplement):68.23. Paintlia AS, Paintlia MK, Mohan S, Singh AK, Singh I. 2013; AMP-activated protein kinase signaling protects oligodendrocytes that restore central nervous system functions in an experimental autoimmune encephalomyelitis model. Am J Pathol. 183:526–41. DOI: 10.1016/j.ajpath.2013.04.030. PMID: 23759513. PMCID: PMC3730772.
Article24. Kim JY, Sohn HJ, Seo JH. 2011; Characterization of the antigenic phenotype of αB-crystallin-expressing peripapillary glial cells in the developing chick retina. Anat Cell Biol. 44:35–40. DOI: 10.5115/acb.2011.44.1.35. PMID: 21519547. PMCID: PMC3080006.
Article25. Wang YH, Li YC, Huo SJ, Yin ZQ. 2012; Alpha-crystallin promotes rat olfactory ensheathing cells survival and proliferation through regulation of PI3K/Akt/mTOR signaling pathways. Neurosci Lett. 531:170–5. DOI: 10.1016/j.neulet.2012.10.057. PMID: 23142719.
Article26. Zotova E, Bharambe V, Cheaveau M, Morgan W, Holmes C, Harris S, Neal JW, Love S, Nicoll JA, Boche D. 2013; Inflammatory components in human Alzheimer's disease and after active amyloid-β42 immunization. Brain. 136(Pt 9):2677–96. DOI: 10.1093/brain/awt210. PMID: 23943781.
Article27. Gupta N, Brown KE, Milam AH. 2003; Activated microglia in human retinitis pigmentosa, late-onset retinal degeneration, and age-related macular degeneration. Exp Eye Res. 76:463–71. DOI: 10.1016/S0014-4835(02)00332-9. PMID: 12634111.
Article28. Biswas SK, Mantovani A. 2010; Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 11:889–96. DOI: 10.1038/ni.1937. PMID: 20856220.
Article29. Narayan DS, Wood JP, Chidlow G, Casson RJ. 2016; A review of the mechanisms of cone degeneration in retinitis pigmentosa. Acta Ophthalmol. 94:748–54. DOI: 10.1111/aos.13141. PMID: 27350263.
Article30. Jin Q, Cheng J, Liu Y, Wu J, Wang X, Wei S, Zhou X, Qin Z, Jia J, Zhen X. 2014; Improvement of functional recovery by chronic metformin treatment is associated with enhanced alternative activation of microglia/macrophages and increased angiogenesis and neurogenesis following experimental stroke. Brain Behav Immun. 40:131–42. DOI: 10.1016/j.bbi.2014.03.003. PMID: 24632338.
Article31. Tayara K, Espinosa-Oliva AM, García-Domínguez I, Ismaiel AA, Boza-Serrano A, Deierborg T, Machado A, Herrera AJ, Venero JL, de Pablos RM. 2018; Divergent effects of metformin on an inflammatory model of Parkinson's disease. Front Cell Neurosci. 12:440. DOI: 10.3389/fncel.2018.00440. PMID: 30519161. PMCID: PMC6258993.
Article32. Means JC, Lopez AA, Koulen P. 2020; Resveratrol protects optic nerve head astrocytes from oxidative stress-induced cell death by preventing caspase-3 activation, Tau dephosphorylation at Ser422 and formation of misfolded protein aggregates. Cell Mol Neurobiol. 40:911–26. DOI: 10.1007/s10571-019-00781-6. PMID: 31919747.
Article33. Eltony SA, Abdelhameed SY. 2017; Effect of chronic administration of sildenafil citrate (Viagra) on the histology of the retina and optic nerve of adult male rat. Tissue Cell. 49(2 Pt B):323–35. DOI: 10.1016/j.tice.2017.01.006. PMID: 28237322.
Article34. Kraig RP, Chesler M. 1990; Astrocytic acidosis in hyperglycemic and complete ischemia. J Cereb Blood Flow Metab. 10:104–14. DOI: 10.1038/jcbfm.1990.13. PMID: 2298827. PMCID: PMC3047406.
Article35. Fenner D, Odili S, Hong HK, Kobayashi Y, Kohsaka A, Siepka SM, Vitaterna MH, Chen P, Zelent B, Grimsby J, Takahashi JS, Matschinsky FM, Bass J. 2011; Generation of N-ethyl-N-nitrosourea (ENU) diabetes models in mice demonstrates genotype-specific action of glucokinase activators. J Biol Chem. 286:39560–72. DOI: 10.1074/jbc.M111.269100. PMID: 21921030. PMCID: PMC3234779.
Article36. Bentley L, Esapa CT, Nesbit MA, Head RA, Evans H, Lath D, Scudamore CL, Hough TA, Podrini C, Hannan FM, Fraser WD, Croucher PI, Brown MA, Brown SD, Cox RD, Thakker RV. 2014; An N-ethyl-N-nitrosourea induced corticotropin-releasing hormone promoter mutation provides a mouse model for endogenous glucocorticoid excess. Endocrinology. 155:908–22. DOI: 10.1210/en.2013-1247. PMID: 24302625. PMCID: PMC4192286.
Article37. Sandell JH, Peters A. 2001; Effects of age on nerve fibers in the rhesus monkey optic nerve. J Comp Neurol. 429:541–53. DOI: 10.1002/1096-9861(20010122)429:4<541::AID-CNE3>3.0.CO;2-5. PMID: 11135234.
Article38. Vinores SA, Herman MM. 1993; Phagocytosis of myelin by astrocytes in explants of adult rabbit cerebral white matter maintained on Gelfoam matrix. J Neuroimmunol. 43:169–76. DOI: 10.1016/0165-5728(93)90088-G. PMID: 8458985.
Article39. Narciso MS, Hokoç JN, Martinez AM. 2001; Watery and dark axons in Wallerian degeneration of the opossum's optic nerve: different patterns of cytoskeletal breakdown? An Acad Bras Cienc. 73:231–43. DOI: 10.1590/S0001-37652001000200008. PMID: 11404785.
Article40. Jones RS, Minogue AM, Connor TJ, Lynch MA. 2013; Amyloid-β-induced astrocytic phagocytosis is mediated by CD36, CD47 and RAGE. J Neuroimmune Pharmacol. 8:301–11. DOI: 10.1007/s11481-012-9427-3. PMID: 23238794.
Article41. Blázquez C, Geelen MJ, Velasco G, Guzmán M. 2001; The AMP-activated protein kinase prevents ceramide synthesis de novo and apoptosis in astrocytes. FEBS Lett. 489:149–53. DOI: 10.1016/S0014-5793(01)02089-0. PMID: 11165240.
Article42. Gabryel B, Liber S. 2018; Metformin limits apoptosis in primary rat cortical astrocytes subjected to oxygen and glucose deprivation. Folia Neuropathol. 56:328–36. DOI: 10.5114/fn.2018.80866. PMID: 30786670.
Article