Developing a Framework for Pandemic COVID-19 Vaccine Allocation: a Modified Delphi Consensus Study in Korea
- Affiliations
-
- 1Department of Internal Medicine, International St. Mary's Hospital, Catholic Kwandong University College of Medicine, Incheon, Korea
- 2Department of Internal Medicine, Korea University Ansan Hospital, Korea University College of Medicine, Seoul, Korea
- 3Department of Internal Medicine, Korea University Guro Hospital, Korea University College of Medicine, Seoul, Korea
- 4Department of Internal Medicine, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea
- 5Department of Pediatrics, St. Vincent's Hospital, College of Medicine, The Catholic University of Korea, Suwon, Korea
- 6Department of Pediatrics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 7Department of Pediatrics, Chungnam National University Hospital, Chungnam National University, Daejeon, Korea
- 8Department of Social and Preventive Medicine, Hallym University College of Medicine, Chuncheon, Korea
- 9Department of Preventive Medicine, Graduate Program in System Health Science and Engineering, Ewha Womans University College of Medicine, Seoul, Korea
- 10Center for Preventive Medicine and Public Health, Seoul National University Bundang Hospital, Seongnam, Korea
- 11Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea
- 12Department of Internal Medicine, Seoul St. Mary's Hospital, The Catholic University College of Medicine, Seoul, Korea
- 13Department of Internal Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- KMID: 2516680
- DOI: http://doi.org/10.3346/jkms.2021.36.e166
Abstract
- Background
This study presents a framework for determining the allocation and distribution of the limited amount of vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
Methods
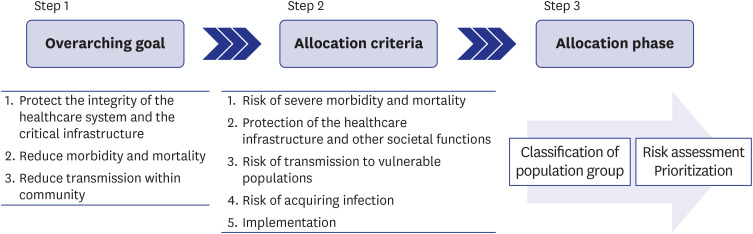
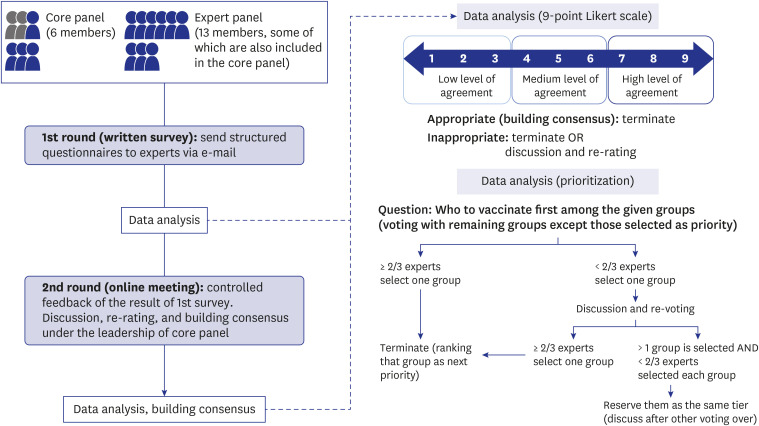
After analyzing the pandemic strategies of the major organizations and countries and with a literature review conducted by a core panel, a modified Delphi survey was administered to 13 experts in the fields of vaccination, infectious disease, and public health in the Republic of Korea. The following topics were discussed: 1) identifying the objectives of the vaccination strategy, 2) identifying allocation criteria, and 3) establishing a step-bystep vaccination framework and prioritization strategy based on the allocation criteria. Two rounds of surveys were conducted for each topic, with a structured questionnaire provided via e-mail in the first round. After analyzing the responses, a meeting with the experts was held to obtain consensus on how to prioritize the population groups.
Results
The first objective of the vaccination strategy was maintenance of the integrity of the healthcare system and critical infrastructure, followed by reduction of morbidity and mortality and reduction of community transmission. In the initial phase, older adult residents in care homes, high-risk health and social care workers, and personal support workers who work in direct contact with coronavirus disease 2019 (COVID-19) patients would be prioritized. Expansion of vaccine supply would allow immunization of older adults not included in phase 1, followed by healthcare workers not previously included and individuals with comorbidities. Further widespread vaccine supply would ensure availability to the extended adult age groups (50–64 years old), critical workers outside the health sector, residents who cannot socially distance, and, eventually, the remaining populations.
Conclusion
This survey provides the much needed insight into the decision-making process for vaccine allocation at the national level. However, flexibility in adapting to strategies will be essential, as new information is constantly emerging.
Figure
Cited by 4 articles
-
Pediatric and Parents' Attitudes Towards COVID-19 Vaccines and Intention to Vaccinate for Children
Soo-Han Choi, Yoon Hee Jo, Kyo Jin Jo, Su Eun Park
J Korean Med Sci. 2021;36(31):e227. doi: 10.3346/jkms.2021.36.e227.Why Fast COVID-19 Vaccination Needed for People with Disabilities and Autistics in Korea?
Wn-Ho Yoon
J Korean Med Sci. 2021;36(37):e267. doi: 10.3346/jkms.2021.36.e267.Safety Monitoring after the BNT162b2 COVID-19 Vaccine among Adults Aged 75 Years or Older
Youn Young Choi, Min-Kyung Kim, Hyeok Choon Kwon, Gunn Hee Kim
J Korean Med Sci. 2021;36(45):e318. doi: 10.3346/jkms.2021.36.e318.SARS-CoV-2 Delta Variant Breakthrough Infection and Onward Secondary Transmission in Household
Seonju Yi, Jong Mu Kim, Young June Choe, Sujin Hong, Siwon Choi, Seong Bae Ahn, Miya Kim, Young-Joon Park
J Korean Med Sci. 2021;37(1):e12. doi: 10.3346/jkms.2022.37.e12.
Reference
-
1. World Health Organization. Weekly epidemiological update on COVID-19 - 6 April 2021. Updated April 4, 2021. Accessed April 6, 2021. https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---6-april-2021.2. European Centre for Disease Prevention and Control. Rapid risk assessment: coronavirus disease 2019 (COVID-19) in the EU/EEA and the UK – eighth update. 8 April 2020. Updated April 8, 2020. Accessed November 29, 2020. https://www.ecdc.europa.eu/sites/default/files/documents/covid-19-rapid-risk-assessment-coronavirus-disease-2019-eighth-update-8-april-2020.pdf.3. Centers for Disease Control and Prevention. COVID data tracker. Nationwide commercial laboratory seroprevalence survey. Accessed March 2, 2021. https://covid.cdc.gov/covid-data-tracker/#national-lab.4. European Centre for Disease Prevention and Control. Immune responses and immunity to SARS-CoV-2. Accessed November 29, 2020. https://www.ecdc.europa.eu/en/covid-19/latest-evidence/immune-responses.5. Rostami A, Sepidarkish M, Leeflang MM, Riahi SM, Nourollahpour Shiadeh M, Esfandyari S, et al. SARS-CoV-2 seroprevalence worldwide: a systematic review and meta-analysis. Clin Microbiol Infect. 2021; 27(3):331–340. PMID: 33228974.
Article6. Korea Disease Control and Prevention Agency. COVID-19 outbreak in Korea. Updated November 23, 2020. Accessed November 29, 2020. http://ncov.mohw.go.kr/tcmBoardView.do?brdId=&brdGubun=&dataGubun=&ncvContSeq=361276&contSeq=361276&board_id=140&gubun=BDJ.7. Noh JY, Seo YB, Yoon JG, Seong H, Hyun H, Lee J, et al. Seroprevalence of anti-SARS-CoV-2 antibodies among outpatients in southwestern Seoul, Korea. J Korean Med Sci. 2020; 35(33):e311. PMID: 32830472.
Article8. World Health Organization. Draft landscape and tracker of COVID-19 candidate vaccines. Updated April 2, 2021. Accessed April 4, 2021. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines.9. Thomas DW, Burns J, Audette J, Carroll A, Dow-Hygelund C, Hay M. 2016.10. Centers for Disease Control and Prevention. Interim updated planning guidance on allocating and targeting pandemic influenza vaccine during an influenza pandemic. Accessed March 2, 2021. https://www.cdc.gov/flu/pandemic-resources/national-strategy/planning-guidance/index.html.11. Kim WJ. Development of policy and strategy for novel influenza A (H1N1) vaccination. Updated 2010. Accessed December 6, 2020. https://scienceon.kisti.re.kr/srch/selectPORSrchReport.do?cn=TRKO201300000256.12. Centers for Disease Control and Prevention. Risk for COVID-19 infection, hospitalization, and death by age group. Updated 2020. Accessed December 6, 2020. https://www.cdc.gov/coronavirus/2019-ncov/covid-data/investigations-discovery/hospitalization-death-by-age.html.13. Korea Disease Control and Prevention Agency. Occurrence status of COVID-19 in South Korea. Updated 2020. Accessed December 6, 2020. http://ncov.mohw.go.kr/bdBoardList_Real.do?brdId=1&brdGubun=11&ncvContSeq=&contSeq=&board_id=&gubun.14. World Health Organization. A global framework to ensure equitable and fair allocation of COVID-19 products and potential implication for COVID-19 vaccines. WHO Member States Briefing. Updated June 18, 2020. Accessed November 29, 2020. https://www.ccghr.ca/wp-content/uploads/2020/06/Global-Allocation-Framework.pdf.15. Mbaeyi S. ACIP COVID-19 Vaccines Work Group. Considerations for COVID-19 vaccine prioritization. Updated June 24, 2020. Accessed November 29, 2020. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2020-06/COVID-08-Mbaey-508.pdf.16. Independent report. Joint Committee on Vaccination and Immunisation: interim advice on priority groups for covid-19 vaccination. Updated June 18, 2020. Accessed November 29, 2020. https://www.gov.uk/government/publications/priority-groups-for-coronavirus-covid-19-vaccination-advice-from-the-jcvi/interim-advice-on-priority-groups-for-covid-19-vaccination.17. World Health Organization. WHO target product profiles for COVID-19 vaccines. Updated April 9, 2020. Accessed February 14, 2021. https://www.who.int/publications/m/item/who-target-product-profiles-for-covid-19-vaccines.18. Gayle H, Foege W, Brown L, Kahn B. Framework for equitable allocation of COVID-19 vaccine. Updated 2020. Accessed March 2, 2021. https://www.nap.edu/catalog/25917/framework-for-equitable-allocation-of-covid-19-vaccine.19. Independent report. JCVI: updated interim advice on priority groups for COVID-19 vaccination. Updated September 25, 2020. Accessed February 11, 2021. https://www.gov.uk/government/publications/priority-groups-for-coronavirus-covid-19-vaccination-advice-from-the-jcvi-25-september-2020/jcvi-updated-interim-advice-on-priority-groups-for-covid-19-vaccination.20. Oliver S. ACIP COVID-19 Vaccines Work Group. Overview of vaccine equity and prioritization frameworks. Updated September 22, 2020. Accessed November 29, 2020. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2020-09/COVID-06-Oliver-508.pdf.21. European Centre for Disease Prevention and Control. Key aspects regarding the introduction and prioritisation of COVID-19 vaccination in the EU/EEA and the UK. Updated October 26, 2020. Accessed February 11, 2021. https://www.ecdc.europa.eu/en/publications-data/key-aspects-regarding-introduction-and-prioritisation-covid-19-vaccination.22. Toner E, Barnill A, Krubiner C, Bernstein J, Privor-Dumm L, Watson M. Interim Framework for COVID-19 Vaccine Allocation and Distribution in the United States. Baltimore, MD, USA: Johns Hopkins Center for Health Security;Updated August 2020. Accessed March 2, 2021. https://www.centerforhealthsecurity.org/our-work/publications/interim-framework-for-covid-19-vaccine-allocation-and-distribution-in-the-us.23. World Health Organization. WHO SAGE values framework for the allocation and prioritization of COVID-19 vaccination. Updated September 14, 2020. Accessed March 2, 2021. https://www.who.int/publications/i/item/who-sage-values-framework-for-the-allocation-and-prioritization-of-covid-19-vaccination.24. World Health Organization. WHO recommendations on pandemic (H1N1) 2009 vaccines. Updated July 13, 2009. Accessed February 7, 2021. https://www.who.int/csr/disease/swineflu/notes/h1n1_vaccine_20090713/en/.25. Mbaeyi S. ACIP COVID-19 Vaccines Work Group. COVID-19 vaccine prioritization: work group considerations. Update July 29, 2020. Accessed November 29, 2020. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2020-07/COVID-07-Mbaeyi-508.pdf.26. Dooling K. ACIP COVID-19 Vaccines Work Group. COVID-19 vaccine prioritization: work group considerations. Updated August 26, 2020. Accessed November 29, 2020. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2020-08/COVID-08-Dooling.pdf.27. Kim SY, Choi MY, Shin SS, Ji SM, Park JJ, Yoo JH, et al. NECA's Handbook for Clinical Practice Guideline Development. Seoul, Korea: National Evidence-based Healthcare Collaborating Agency;2015.28. Choi MJ, Song JY, Noh JY, Yoon JG, Lee SN, Heo JY, et al. Disease burden of hospitalized community-acquired pneumonia in South Korea: Analysis based on age and underlying medical conditions. Medicine (Baltimore). 2017; 96(44):e8429. PMID: 29095281.29. Ji W, Huh K, Kang M, Hong J, Bae GH, Lee R, et al. Effect of underlying comorbidities on the infection and severity of COVID-19 in Korea: a nationwide case-control study. J Korean Med Sci. 2020; 35(25):e237. PMID: 32597048.
Article30. World Health Organization. Fair allocation mechanism for COVID-19 vaccines through the COVAX Facility. Final working version. Updated September 9, 2020. Accessed March 2, 2021. https://www.who.int/publications/m/item/fair-allocation-mechanism-for-covid-19-vaccines-through-the-covax-facility.31. European Commission. EU vaccines strategy. Accessed February 12, 2021. https://ec.europa.eu/info/live-work-travel-eu/coronavirus-response/public-health/coronavirus-vaccines-strategy_en.32. Australian Technical Advisory Group on Immunisation (ATAGI). Preliminary advice on general principles to guide the prioritisation of target populations in a COVID-19 vaccination program in Australia. Updated November 13, 2020. Accessed February 12, 2021. https://www.health.gov.au/resources/publications/atagi-preliminary-advice-on-general-principles-to-guide-the-prioritisation-of-target-populations-in-a-covid-19-vaccination-program-in-australia.33. National Advisory Committee on Immunization (NACI). Guidance on the prioritization of initial doses of COVID-19 vaccine(s). Accessed February 12, 2021. https://www.canada.ca/en/public-health/services/immunization/national-advisory-committee-on-immunization-naci/guidance-prioritization-initial-doses-covid-19-vaccines.html.34. Joint Committee on Vaccination and Immunisation (JCVI). Independent report. Priority groups for coronavirus (COVID-19) vaccination: advice from the JCVI, 30 December 2020. Update December 30, 2020. Accessed February 12, 2021. https://www.gov.uk/government/publications/priority-groups-for-coronavirus-covid-19-vaccination-advice-from-the-jcvi-30-december-2020.35. Centers for Disease Control and Prevention. COVID-19, people at increased risk and other people who need to take extra precautions. Accessed October 30, 2020. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/index.html.36. Sung HK, Kim JY, Heo J, Seo H, Jang YS, Kim H, et al. Clinical course and outcomes of 3,060 patients with coronavirus disease 2019 in Korea, January–May 2020. J Korean Med Sci. 2020; 35(30):e280. PMID: 32743995.
Article37. Viboud C, Boëlle PY, Cauchemez S, Lavenu A, Valleron AJ, Flahault A, et al. Risk factors of influenza transmission in households. Br J Gen Pract. 2004; 54(506):684–689. PMID: 15353055.
Article38. Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021; 384(5):403–416. PMID: 33378609.
Article39. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020; 383(27):2603–2615. PMID: 33301246.
Article40. Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021; 397(10269):99–111. PMID: 33306989.41. The first interim data analysis of the Sputnik V vaccine against COVID-19 phase III clinical trials in the Russian Federation demonstrated 92% efficacy [press release]. Updated November 11, 2020. Accessed February 14, 2021. https://sputnikvaccine.com/newsroom/pressreleases/the-first-interim-data-analysis-of-the-sputnik-v-vaccine-against-covid-19-phase-iii-clinical-trials-/.42. Novavax. Novavax COVID-19 vaccine demonstrates 89.3% efficacy in UK phase 3 trial [press release]. Updated January 29, 2021. Accessed February 14, 2021. https://ir.novavax.com/news-releases/news-release-details/novavax-covid-19-vaccine-demonstrates-893-efficacy-uk-phase-3.43. Statistics Korea. Population sector. Accessed February 11, 2021. https://kosis.kr/statisticsList/statisticsListIndex.do?menuId=M_01_01&vwcd=MT_ZTITLE&parmTabId=M_01_01&outLink=Y&entrType=#A11_2015_1_10_10.6.44. Ministry of Health and Welfare, Social Welfare Facility Information System. The status of facility residents. Accessed October 27, 2020. http://www.w4c.go.kr/intro/introFcltInmtSttus.do.45. Ministry of Education. Kindergarten, elementary, middle, and high school education statistics for 2020. Accessed October 27, 2020. https://kess.kedi.re.kr/index.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Why Fast COVID-19 Vaccination Needed for People with Disabilities and Autistics in Korea?
- Delphi Survey for COVID-19 Vaccination in Korean Children Between 5 and 11 Years Old
- Expert Consensus on COVID-19 Vaccination in Korean Adolescents: A Modified Delphi Survey
- Current updates and research on plant-based vaccines for coronavirus disease 2019
- Evidence-Developing Disease Control of Coronavirus Disease 2019