Clin Endosc.
2021 May;54(3):363-370. 10.5946/ce.2020.058.
Reduced Intravenous Fluorescein Dose for Upper and Lower Gastrointestinal Tract Probe-Based Confocal Laser Endomicroscopy
- Affiliations
-
- 1Endoscopy Division, National Cancer Center Hospital, Tokyo, Japan
- 2Division of Gastroenterology, Department of Medicine, Showa University School of Medicine, Tokyo, Japan
- 3Department of Gastroenterology, Keiyu Hospital, Kanagawa, Japan
- 4Department of Gastroenterology, Arao Municipal Hospital, Kumamoto, Japan
- 5Division of Gastroenterology and Hepatology, Toho University Medical Center Ohashi Hospital, Tokyo, Japan
- 6Division of Gastroenterology and Hepatology, Department of Medicine, Nihon University School of Medicine, Tokyo, Japan
- 7Department of Gastroenterology, Chofu Touzan Hospital, Tokyo, Japan
- 8Division of Gastroenterology, Cathay General Hospital, Taipei, Taiwan
- 9Cancer Screening Center, National Cancer Center Hospital, Tokyo, Japan
- KMID: 2516316
- DOI: http://doi.org/10.5946/ce.2020.058
Abstract
- Background/Aims
Probe-based confocal laser endomicroscopy (pCLE) requires the administration of intravenous (IV) fluorescein. This study aimed to determine the optimal dose of IV fluorescein for both upper and lower gastrointestinal (GI) tract pCLE.
Methods
Patients 20 to 79 years old with gastric high-grade dysplasia (HGD) or colorectal neoplasms (CRNs) were enrolled in the study. The dose de-escalation method was employed with five levels. The primary endpoint of the study was the determination of the optimal dose of IV fluorescein for pCLE of the GI tract. The reduced dose was determined based on off-line reviews by three endoscopists. An insufficient dose of fluorescein was defined as the dose of fluorescein with which the pCLE images were not deemed to be visible. If all three endoscopists determined that the tissue structure was visible, the doses were de-escalated.
Results
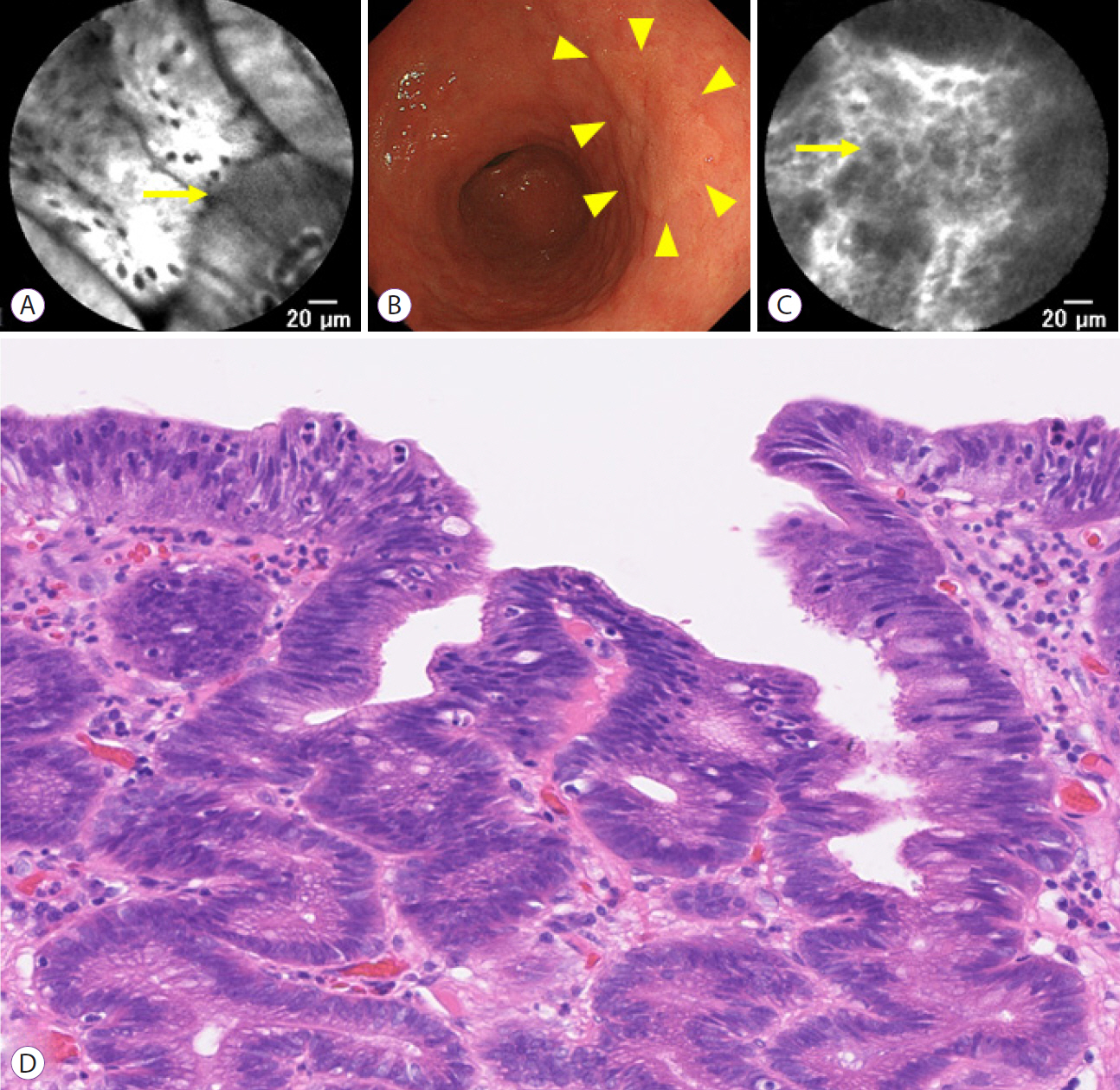
A total of 12 patients with gastric HGD and 12 patients with CRNs were enrolled in the study. Doses were de-escalated to 0.5 mg/kg of fluorescein for both non-neoplastic duodenal and colorectal mucosa. All gastric HGD or CRNs were visible with pCLE with IV fluorescein at 0.5 mg/kg.
Conclusions
In the present study, pCLE with IV fluorescein 0.5 mg/kg was adequate to visualize the magnified structure of both the upper and lower GI tract.
Keyword
Figure
Reference
-
1. Wallace MB, Fockens P. Probe-based confocal laser endomicroscopy. Gastroenterology. 2009; 136:1509–1513.
Article2. Sharma P, Meining AR, Coron E, et al. Real-time increased detection of neoplastic tissue in Barrett’s esophagus with probe-based confocal laser endomicroscopy: final results of an international multicenter, prospective, randomized, controlled trial. Gastrointest Endosc. 2011; 74:465–472.
Article3. Park JC, Park Y, Kim HK, et al. Probe-based confocal laser endomicroscopy in the margin delineation of early gastric cancer for endoscopic submucosal dissection. J Gastroenterol Hepatol. 2017; 32:1046–1054.
Article4. Kim B, Kim YH, Park SJ, et al. Probe-based confocal laser endomicroscopy for evaluating the submucosal invasion of colorectal neoplasms. Surg Endosc. 2017; 31:594–601.
Article5. Abe S, Saito Y, Oono Y, et al. Pilot study on probe-based confocal laser endomicroscopy for colorectal neoplasms: an initial experience in Japan. Int J Colorectal Dis. 2018; 33:1071–1078.
Article6. Buchner AM, Gomez V, Heckman MG, et al. The learning curve of in vivo probe-based confocal laser endomicroscopy for prediction of colorectal neoplasia. Gastrointest Endosc. 2011; 73:556–560.
Article7. Pittayanon R, Rerknimitr R, Wisedopas N, et al. The learning curve of gastric intestinal metaplasia interpretation on the images obtained by probe-based confocal laser endomicroscopy. Diagn Ther Endosc. 2012; 2012:278045.
Article8. Wallace MB, Meining A, Canto MI, et al. The safety of intravenous fluorescein for confocal laser endomicroscopy in the gastrointestinal tract. Aliment Pharmacol Ther. 2010; 31:548–552.
Article9. Ha SO, Kim DY, Sohn CH, Lim KS. Anaphylaxis caused by intravenous fluorescein: clinical characteristics and review of literature. Intern Emerg Med. 2014; 9:325–330.
Article10. Xu K, Tzankova V, Li C, Sharma S. Intravenous fluorescein angiography-associated adverse reactions. Can J Ophthalmol. 2016; 51:321–325.
Article11. Marcus DF, Bovino JA, Williams D. Adverse reactions during intravenous fluorescein angiography. Arch Ophthalmol. 1984; 102:825.
Article12. Yannuzzi LA, Rohrer KT, Tindel LJ, et al. Fluorescein angiography complication survey. Ophthalmology. 1986; 93:611–617.
Article13. Lira RP, Oliveira CL, Marques MV, Silva AR, Pessoa Cde C. Adverse reactions of fluorescein angiography: a prospective study. Arq Bras Oftalmol. 2007; 70:615–618.
Article14. McLauchlan R, Waterman H, Waterman C, Hillier V, Dodd C. Ethnic variation in fluorescein angiography induced nausea and vomiting. Eye (Lond). 2001; 15(Pt 2):159–162.
Article15. Keerl R, Weber RK, Draf W, Wienke A, Schaefer SD. Use of sodium fluorescein solution for detection of cerebrospinal fluid fistulas: an analysis of 420 administrations and reported complications in Europe and the United States. Laryngoscope. 2004; 114:266–272.
Article16. Dowlati A, Robertson K, Radivoyevitch T, et al. Novel phase I dose de-escalation design trial to determine the biological modulatory dose of the antiangiogenic agent SU5416. Clin Cancer Res. 2005; 11:7938–7944.
Article17. Sano Y, Tanaka S, Kudo SE, et al. Narrow-band imaging (NBI) magnifying endoscopic classification of colorectal tumors proposed by the Japan NBI expert team. Dig Endosc. 2016; 28:526–533.
Article18. Wallace M, Lauwers GY, Chen Y, et al. Miami classification for probe-based confocal laser endomicroscopy. Endoscopy. 2011; 43:882–891.
Article19. Zhang YL, Bai L, Li Z, et al. A lower dose of fluorescein sodium is more suitable for confocal laser endomicroscopy: a feasibility study. Gastrointest Endosc. 2016; 84:917–923.e5.
Article20. Caillol F, Bories E, Poizat F, et al. Endomicroscopy in bile duct: inflammation interferes with pCLE applied in the bile duct: a prospective study of 54 patients. United European Gastroenterol J. 2013; 1:120–127.
Article21. Shahid MW, Crook JE, Meining A, et al. Exploring the optimal fluorescein dose in probe-based confocal laser endomicroscopy for colonic imaging. J Interv Gastroenterol. 2011; 1:166–171.22. Nonaka K, Ohata K, Nakai Y. Probe-based confocal laser endomicroscopy of the duodenal mucosa with fluorescein dispersion. Dig Endosc. 2014; 26:604.
Article23. Nonaka K, Ohata K, Ban S, et al. Histopathological confirmation of similar intramucosal distribution of fluorescein in both intravenous administration and local mucosal application for probe-based confocal laser endomicroscopy of the normal stomach. World J Clin Cases. 2015; 3:993–999.
Article24. Tashima T, Nonaka K, Ryozawa S, Tanisaka Y, Fujino T. Successful ex-vivo probe-based confocal laser endomicroscopy of a superficial nonampullary duodenal epithelial tumor with dripping of food additives: a case of tubular adenocarcinoma. VideoGIE. 2019; 4:128–130.
Article25. Meining A, Shah RJ, Slivka A, et al. Classification of probe-based confocal laser endomicroscopy findings in pancreaticobiliary strictures. Endoscopy. 2012; 44:251–257.
Article26. Spessotto P, Fornasarig M, Pivetta E, et al. Probe-based confocal laser endomicroscopy for in vivo evaluation of the tumor vasculature in gastric and rectal carcinomas. Sci Rep. 2017; 7:9819.
Article27. Tian Y, Zheng Y, Dong J, Zhang J, Wang H. Papaverine adjuvant therapy for microcirculatory disturbance in severe ulcerative colitis complicated with CMV infection: a case report. Clin J Gastroenterol. 2019; 12:407–413.
Article28. Shah T, Lippman R, Kohli D, Mutha P, Solomon S, Zfass A. Accuracy of probe-based confocal laser endomicroscopy (pCLE) compared to random biopsies during endoscopic surveillance of Barrett’s esophagus. Endosc Int Open. 2018; 6:E414–E420.
Article29. Wellikoff AS, Holladay RC, Downie GH, Chaudoir CS, Brandi L, Turbat-Herrera EA. Comparison of in vivo probe-based confocal laser endomicroscopy with histopathology in lung cancer: a move toward optical biopsy. Respirology. 2015; 20:967–974.
Article30. Caillol F, Bories E, Autret A, et al. Evaluation of pCLE in the bile duct: final results of EMID study: pCLE: impact in the management of bile duct strictures. Surg Endosc. 2015; 29:2661–2668.31. Löhr JM, Lönnebro R, Stigliano S, et al. Outcome of probe-based confocal laser endomicroscopy (pCLE) during endoscopic retrograde cholangiopancreatography: a single-center prospective study in 45 patients. United European Gastroenterol J. 2015; 3:551–560.
Article32. Wu K, Liu JJ, Adams W, et al. Dynamic real-time microscopy of the urinary tract using confocal laser endomicroscopy. Urology. 2011; 78:225–231.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Confocal Laser Endomicroscopy and Molecular Imaging in Barrett Esophagus and Stomach
- High-Resolution Probe-Based Confocal Laser Endomicroscopy for Diagnosing Biliary Diseases
- A Review of Probe-Based Confocal Laser Endomicroscopy for Pancreaticobiliary Disease
- Endoscopic ultrasound-guided needle-based confocal laser endomicroscopy for diagnosis of solid pancreatic lesions
- Application and Efficacy of Super-Magnifying Endoscopy for the Lower Intestinal Tract