J Korean Med Sci.
2021 May;36(18):e133. 10.3346/jkms.2021.36.e133.
Empty Sella Syndrome Associated with Growth Hormone Deficiency: the First Case Report of WeissKruszka Syndrome
- Affiliations
-
- 1Department of Pediatrics, Inha University Hospital, Inha University College of Medicine, Incheon, Korea
- 2Northwest Gyeonggi Regional Center for Rare Disease, Inha University Hospital, Incheon, Korea
- 33 Billion Inc., Seoul, Korea
- 4Department of Psychiatry, Inha University Hospital, Inha University College of Medicine, Incheon, Korea
- 5Department of Ophthalmology, Inha University Hospital, Inha University College of Medicine, Incheon, Korea
- KMID: 2515860
- DOI: http://doi.org/10.3346/jkms.2021.36.e133
Abstract
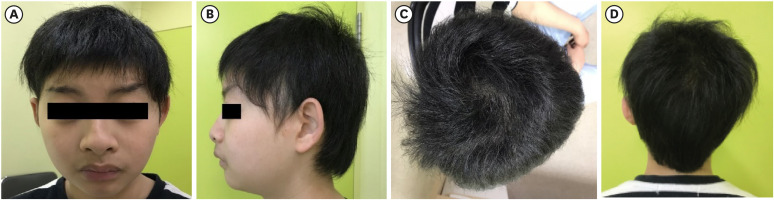
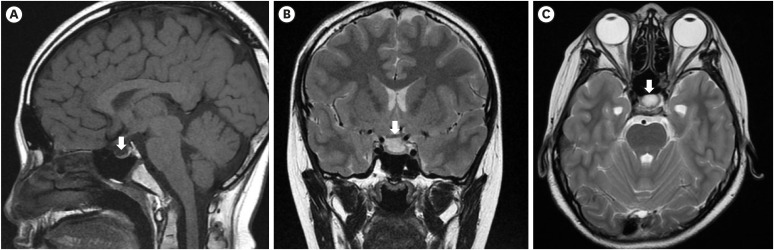
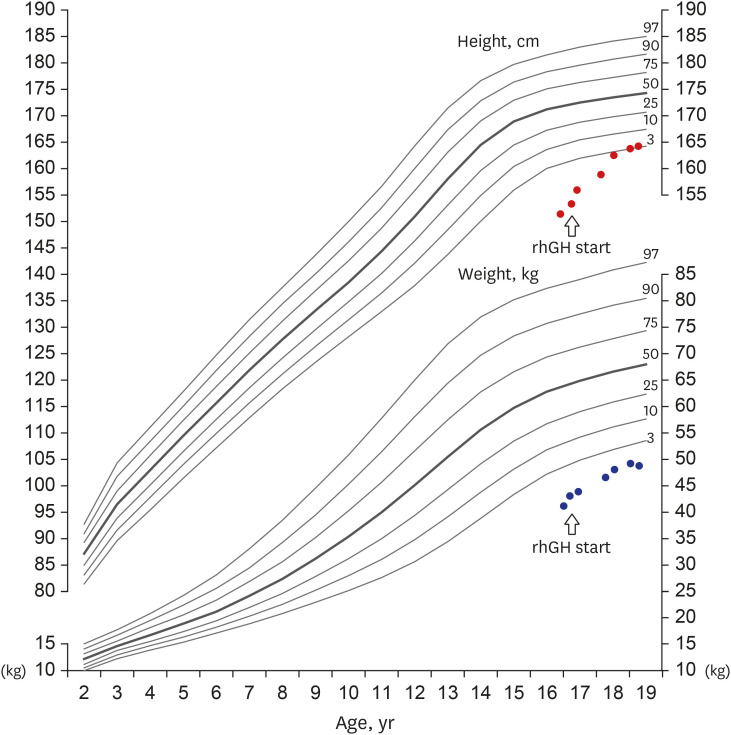
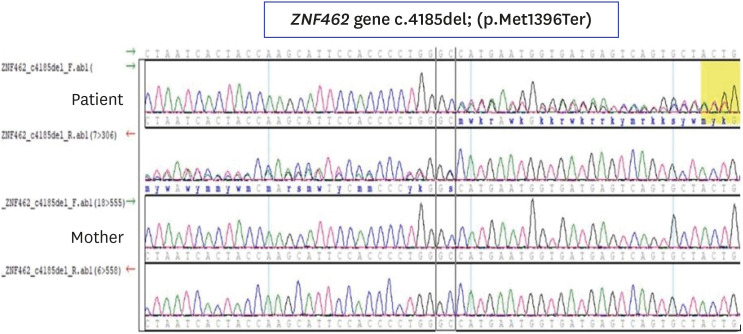
- Weiss-Kruszka syndrome (WSKA), caused by heterozygous loss-of-function variants in ZNF462 gene, is a recently described and extremely rare genetic disorder. The main phenotypes include characteristic craniofacial features, ptosis, dysgenesis of the corpus callosum, and neurodevelopmental impairment. We report the first Korean boy with molecularly confirmed WSKA presenting with an atypical manifestation. A 16-year-old boy with a history of bilateral ptosis surgery presented with short stature (−3.49 standard deviation score) and delayed puberty. The patient showed characteristic craniofacial features including an inverted triangular-shaped head, exaggerated Cupid's bow, arched eyebrows, down-slanting palpebral fissures, and poorly expressive face. He had a mild degree of intellectual disability and mild hypotonia. Endocrine studies in the patient demonstrated complete growth hormone deficiency (GHD) associated with empty sella syndrome (ESS), based on a magnetic resonance imaging study for the brain that showed a flattened pituitary gland and cerebrospinal fluid space herniated into the sella turcica. To identify the genetic cause, we performed whole exome sequencing (WES). Through WES, a novel de novo heterozygous nonsense variant, c.4185del; p.(Met1396Ter) in ZNF462 was identified. This is the first case of WSKA accompanied by primary ESS associated with GHD. More clinical and functional studies are needed to elucidate this association.
Figure
Reference
-
1. González-Tarancón R, Salvador-Rupérez E, Miramar Gallart MD, Barroso E, Díez García-Prieto I, Pérez Delgado R, et al. A novel mutation in the ZNF462 gene c.3306dup; p.(Gln1103Thrfs*10) is associated to Weiss-Kruszka syndrome. A case report. Acta Clin Belg. 2020; 1–4.2. Kruszka P. Weiss-Kruszka syndrome. In : Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Mirzaa G, editors. GeneReviews®. Seattle, WA: University of Washington;1993.3. Nagase T, Nakayama M, Nakajima D, Kikuno R, Ohara O. Prediction of the coding sequences of unidentified human genes. XX. The complete sequences of 100 new cDNA clones from brain which code for large proteins in vitro. DNA Res. 2001; 8(2):85–95. PMID: 11347906.
Article4. Weiss K, Wigby K, Fannemel M, Henderson LB, Beck N, Ghali N, et al. Haploinsufficiency of ZNF462 is associated with craniofacial anomalies, corpus callosum dysgenesis, ptosis, and developmental delay. Eur J Hum Genet. 2017; 25(8):946–951. PMID: 28513610.
Article5. Uliana V, Percesepe A. Reverse phenotyping comes of age. Mol Genet Metab. 2016; 118(4):230–231. PMID: 27211610.
Article6. Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015; 17(5):405–424. PMID: 25741868.
Article7. Cosemans N, Vandenhove L, Maljaars J, Van Esch H, Devriendt K, Baldwin A, et al. ZNF462 and KLF12 are disrupted by a de novo translocation in a patient with syndromic intellectual disability and autism spectrum disorder. Eur J Med Genet. 2018; 61(7):376–383. PMID: 29427787.
Article8. Kruszka P, Hu T, Hong S, Signer R, Cogné B, Isidor B, et al. Phenotype delineation of ZNF462 related syndrome. Am J Med Genet A. 2019; 179(10):2075–2082. PMID: 31361404.9. Nowick K, Gernat T, Almaas E, Stubbs L. Differences in human and chimpanzee gene expression patterns define an evolving network of transcription factors in brain. Proc Natl Acad Sci U S A. 2009; 106(52):22358–22363. PMID: 20007773.
Article10. De Marinis L, Bonadonna S, Bianchi A, Maira G, Giustina A. Primary empty sella. J Clin Endocrinol Metab. 2005; 90(9):5471–5477. PMID: 15972577.
Article11. Lee SS, Han AL, Ahn MB, Kim SH, Cho WK, Cho KS, et al. Growth without growth hormone in combined pituitary hormone deficiency caused by pituitary stalk interruption syndrome. Ann Pediatr Endocrinol Metab. 2017; 22(1):55–59. PMID: 28443260.
Article12. Son HW, Lee JE, Oh SH, Keum C, Chung WY. Effects of long-term growth hormone therapy in a girl with Floating-Harbor syndrome. Ann Pediatr Endocrinol Metab. 2020; 25(2):126–131. PMID: 32615693.
Article13. Chiloiro S, Giampietro A, Bianchi A, Tartaglione T, Capobianco A, Anile C, et al. Diagnosis of endocrine disease: primary empty sella: a comprehensive review. Eur J Endocrinol. 2017; 177(6):R275–85. PMID: 28780516.
Article14. Hayakawa K, Konishi Y, Matsuda T, Kuriyama M, Konishi K, Yamashita K, et al. Development and aging of brain midline structures: assessment with MR imaging. Radiology. 1989; 172(1):171–177. PMID: 2740500.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A case of acromegaly with empty sella syndrome associated with colonic neoplasm
- Isolated adrenocorticotropic hormone deficiency associated with empty sella syndrome
- Two Cases of Graves Disease Associated The Empty Sella Syndrome
- A Case of Primary Empty Sella Syndrome with Central Diabetes Insipidus
- A Case of Empty Sella Syndrome with Cerebrospinal Fluid Rhinorrhea