J Korean Med Sci.
2021 Apr;36(16):e105. 10.3346/jkms.2021.36.e105.
Revised Korean Antiviral Guideline Reduces the Hepatitis B-related Hepatocellular Carcinoma Risk in Cirrhotic Patients
- Affiliations
-
- 1Department of Internal Medicine, Institute of Gastroenterology, Yonsei University College of Medicine, Seoul, Korea
- 2Department of Internal Medicine, Kyungpook National University Hospital, Daegu, Korea
- 3Yonsei Liver Center, Severance Hospital, Seoul, Korea
- 4Division of Biostatistics, Department of Biomedical Systems Informatics, Yonsei University College of Medicine, Seoul, Korea
- 5Biostatistics Collaboration Unit, Department of Biomedical Systems Informatics, Yonsei University College of Medicine, Seoul, Korea
- KMID: 2515086
- DOI: http://doi.org/10.3346/jkms.2021.36.e105
Abstract
- Background
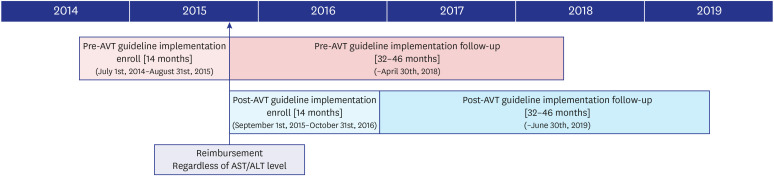
Since September 2015, the initiation of antiviral therapy (AVT) for patients with chronic hepatitis B (CHB)-related cirrhosis has been reimbursed according to the revised Korean Association for the Study of Liver (KASL) guideline, if the patient had hepatitis B virus DNA level ≥ 2,000 IU/L, regardless of aminotransferase or alanine aminotransferase levels. This study investigated whether the KASL guideline implementation reduced the risk of CHB-related hepatocellular carcinoma (HCC) in patients with cirrhosis in South Korea.
Methods
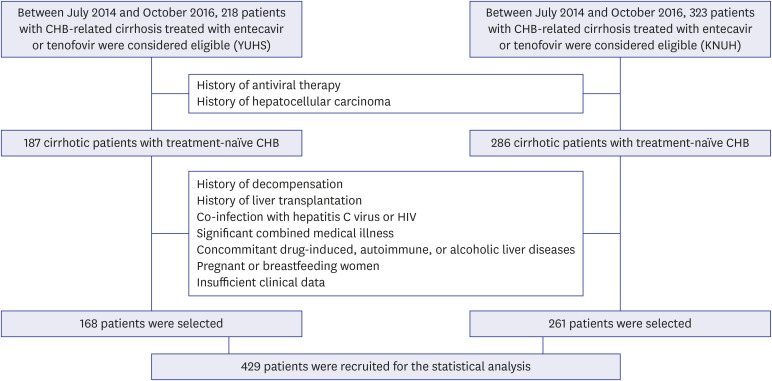
A total of 429 patients with CHB-related cirrhosis who initiated AVT between 2014 and 2016 were recruited. The risk of HCC development was compared between patients who initiated AVT before and after September 2015 (pre-guideline [n = 196, 45.7%] vs. postguideline implementation [n = 233, 54.3%]).
Results
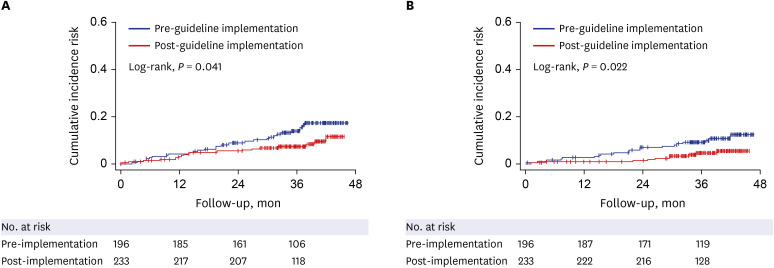
Univariate analysis showed that AVT initiation before guideline implementation, older age, male gender, and diabetes significantly predicted increased risk of HCC development (all P < 0.05). Subsequent multivariate analysis showed that AVT initiation before guideline implementation (HR = 1.941), older age (HR = 5.762), male gender (HR = 2.555), and diabetes (HR = 1.568) independently predicted increased risk of HCC development (all P < 0.05). Additionally, multivariate analysis showed that AVT initiation before guideline implementation (HR = 2.309), male gender (HR = 3.058), and lower platelet count (HR = 0.989) independently predicted mortality (P < 0.05). The cumulative incidences of HCC and mortality were significantly higher in patients who initiated AVT before guideline implementation than in those who initiated AVT after guideline implementation (all P < 0.05, log-rank test).
Conclusion
The prognosis of patients with CHB-related cirrhosis who initiated AVT improved after guideline implementation according to the revised KASL guideline.
Figure
Reference
-
1. World Health Organization. Hepatitis B vaccines: WHO position paper, July 2017 - recommendations. Vaccine. 2019; 37(2):223–225. PMID: 28743487.2. Kim DS, Lim TS, Jeon MY, Kim BK, Park JY, Kim DY, et al. Transarterial chemoembolization in treatment-naïve and recurrent hepatocellular carcinoma: a propensity-matched outcome analysis. Dig Dis Sci. 2019; 64(12):3660–3668. PMID: 31187326.
Article3. Ikeda K, Saitoh S, Suzuki Y, Kobayashi M, Tsubota A, Koida I, et al. Disease progression and hepatocellular carcinogenesis in patients with chronic viral hepatitis: a prospective observation of 2215 patients. J Hepatol. 1998; 28(6):930–938. PMID: 9672166.
Article4. Sookoian S, Pirola CJ. Genetic predisposition in nonalcoholic fatty liver disease. Clin Mol Hepatol. 2017; 23(1):1–12. PMID: 28268262.
Article5. Lai CL, Gane E, Liaw YF, Hsu CW, Thongsawat S, Wang Y, Globe Study Group, et al. Telbivudine versus lamivudine in patients with chronic hepatitis B. N Engl J Med. 2007; 357(25):2576–2588. PMID: 18094378.
Article6. Park MS, Kim BK, Kim KS, Kim JK, Kim SU, Park JY, et al. Antiviral efficacies of currently available rescue therapies for multidrug-resistant chronic hepatitis B. Clin Mol Hepatol. 2013; 19(1):29–35. PMID: 23593607.
Article7. Kim DS, Jeon MY, Lee HW, Kim BK, Park JY, Kim DY, et al. Influence of hepatic steatosis on the outcomes of patients with chronic hepatitis B treated with entecavir and tenofovir. Clin Mol Hepatol. 2019; 25(3):283–293. PMID: 30419649.
Article8. Kim SU, Seo YS, Lee HA, Kim MN, Lee YR, Lee HW, et al. A multicenter study of entecavir vs. tenofovir on prognosis of treatment-naïve chronic hepatitis B in South Korea. J Hepatol. 2019; 71(3):456–464. PMID: 30959156.
Article9. Liang LY, Wong GL. Unmet need in chronic hepatitis B management. Clin Mol Hepatol. 2019; 25(2):172–180. PMID: 30754963.
Article10. Yim HJ, Kim JH, Park JY, Yoon EL, Park H, Kwon JH, et al. Comparison of clinical practice guidelines for the management of chronic hepatitis B: when to start, when to change, and when to stop. Clin Mol Hepatol. 2020; 26(4):411–429. PMID: 32854458.
Article11. McMahon BJ, Bulkow L, Simons B, Zhang Y, Negus S, Homan C, et al. Relationship between level of hepatitis B virus DNA and liver disease: a population-based study of hepatitis B e antigen-negative persons with hepatitis B. Clin Gastroenterol Hepatol. 2014; 12(4):701–706.e1-3. PMID: 24035774.
Article12. Korean Association for the Study of the Liver. Management of chronic hepatitis B. Clin Mol Hepatol. 2012; 18(2):109–162. PMID: 22893865.13. Cristina SJ, Marta CM, Mercedes GS, Almudena PM, Álvaro HM, Luis VS, et al. Characterization and evaluation of liver fibrosis grade in patients with chronic hepatitis B virus infection and normal transaminases. Clin Mol Hepatol. 2018; 24(4):384–391. PMID: 29969885.
Article14. Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology. 2007; 45(2):507–539. PMID: 17256718.
Article15. Liaw YF, Leung N, Kao JH, Piratvisuth T, Gane E, Han KH, et al. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2008 update. Hepatol Int. 2008; 2(3):263–283. PMID: 19669255.
Article16. Wong GL. Management of chronic hepatitis B patients in immunetolerant phase: what latest guidelines recommend. Clin Mol Hepatol. 2018; 24(2):108–113. PMID: 29353469.
Article17. Lee S, Kim BK, Kim SU, Park SY, Kim JK, Lee HW, et al. Clinical outcomes and prognostic factors of patients with advanced hepatocellular carcinoma treated with sorafenib as first-line therapy: a Korean multicenter study. J Gastroenterol Hepatol. 2014; 29(7):1463–1469. PMID: 25273508.
Article18. Betensky RA. Measures of follow-up in time-to-event studies: why provide them and what should they be? Clin Trials. 2015; 12(4):403–408. PMID: 26025565.
Article19. Lai M, Hyatt BJ, Nasser I, Curry M, Afdhal NH. The clinical significance of persistently normal ALT in chronic hepatitis B infection. J Hepatol. 2007; 47(6):760–767. PMID: 17928090.
Article20. Kumar M, Sarin SK, Hissar S, Pande C, Sakhuja P, Sharma BC, et al. Virologic and histologic features of chronic hepatitis B virus-infected asymptomatic patients with persistently normal ALT. Gastroenterology. 2008; 134(5):1376–1384. PMID: 18471514.
Article21. Casado JL. Renal and bone toxicity with the use of tenofovir: understanding at the end. AIDS Rev. 2016; 18(2):59–68. PMID: 27230467.22. Cho H, Cho Y, Cho EJ, Lee JH, Yu SJ, Oh KH, et al. Tenofovir-associated nephrotoxicity in patients with chronic hepatitis B: two cases. Clin Mol Hepatol. 2016; 22(2):286–291. PMID: 27377911.
Article23. Lee SW, Kwon JH, Lee HL, Yoo SH, Nam HC, Sung PS, et al. Comparison of tenofovir and entecavir on the risk of hepatocellular carcinoma and mortality in treatment-naïve patients with chronic hepatitis B in Korea: a large-scale, propensity score analysis. Gut. 2020; 69(7):1301–1308. PMID: 31672838.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Antiviral therapy in chronic hepatitis B: Revised national medical insurance imbursement policy
- Development and surveillance of hepatocellular carcinoma in patients with sustained virologic response after antiviral therapy for chronic hepatitis C
- Who Needs Screening for the Early Detection of Hepatocellular Carcinoma ?
- Long Term Efficacy of Antiviral Therapy: Mortality and Incidence of Hepatocellular Carcinoma
- Treatment of Hepatitis C in Special Conditions: Liver Cirrhosis