Cancer Res Treat.
2021 Apr;53(2):471-479. 10.4143/crt.2020.577.
Targeting Hypoxia Using Evofosfamide and Companion Hypoxia Imaging of FMISO-PET in Advanced Biliary Tract Cancer
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea
- 2Department of Nuclear Medicine, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea
- 3Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea
- KMID: 2514928
- DOI: http://doi.org/10.4143/crt.2020.577
Abstract
- Purpose
Hypoxia is widely known as one of the mechanisms of chemoresistance and as an environmental condition which triggers invasion and metastasis of cancer. Evofosfamide is a hypoxia-activated prodrug of the cytotoxin bromo-isophosphoramide mustard conjugated with 2-nitroimidazole. Biliary tract cancer (BTC) is known to contain large hypoxic area. This study evaluated the efficacy and safety of evofosfamide as a second-line treatment of advanced BTC.
Materials and Methods
Patients received evofosfamide at a dose of 340 mg/m2 on days 1, 8, and 15 of every 28-day cycle. Primary end-point was progression-free survival (PFS) rate at 4-months (4m-PFSR). Secondary end-points included overall survival (OS), PFS, disease control rate (DCR), metabolic response by 18F-fluorodeoxyglucose positron emission tomography (PET), hypoxic parameters evaluated by 18F-fluoromisonidazole (FMISO) PET and toxicity.
Results
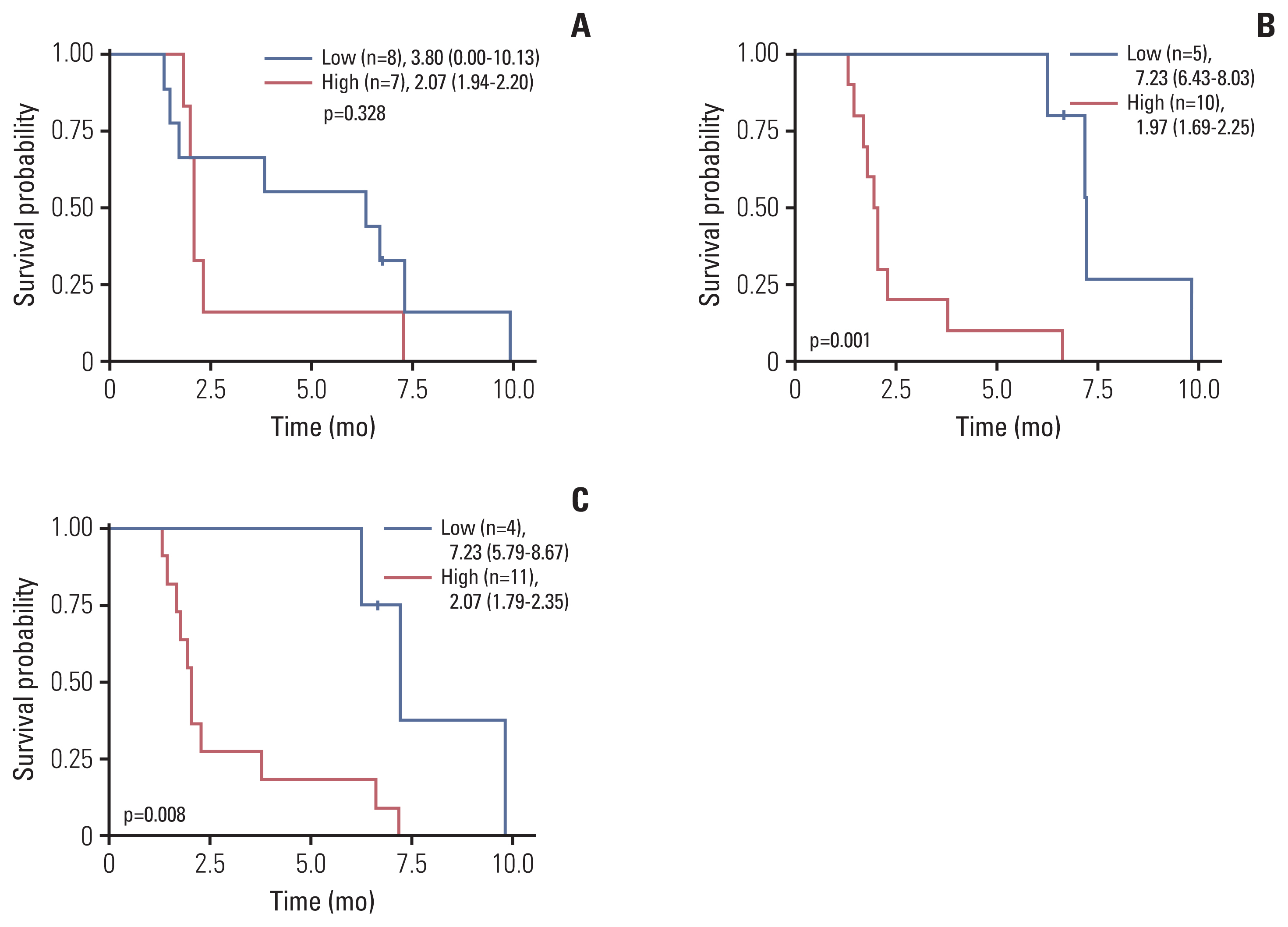
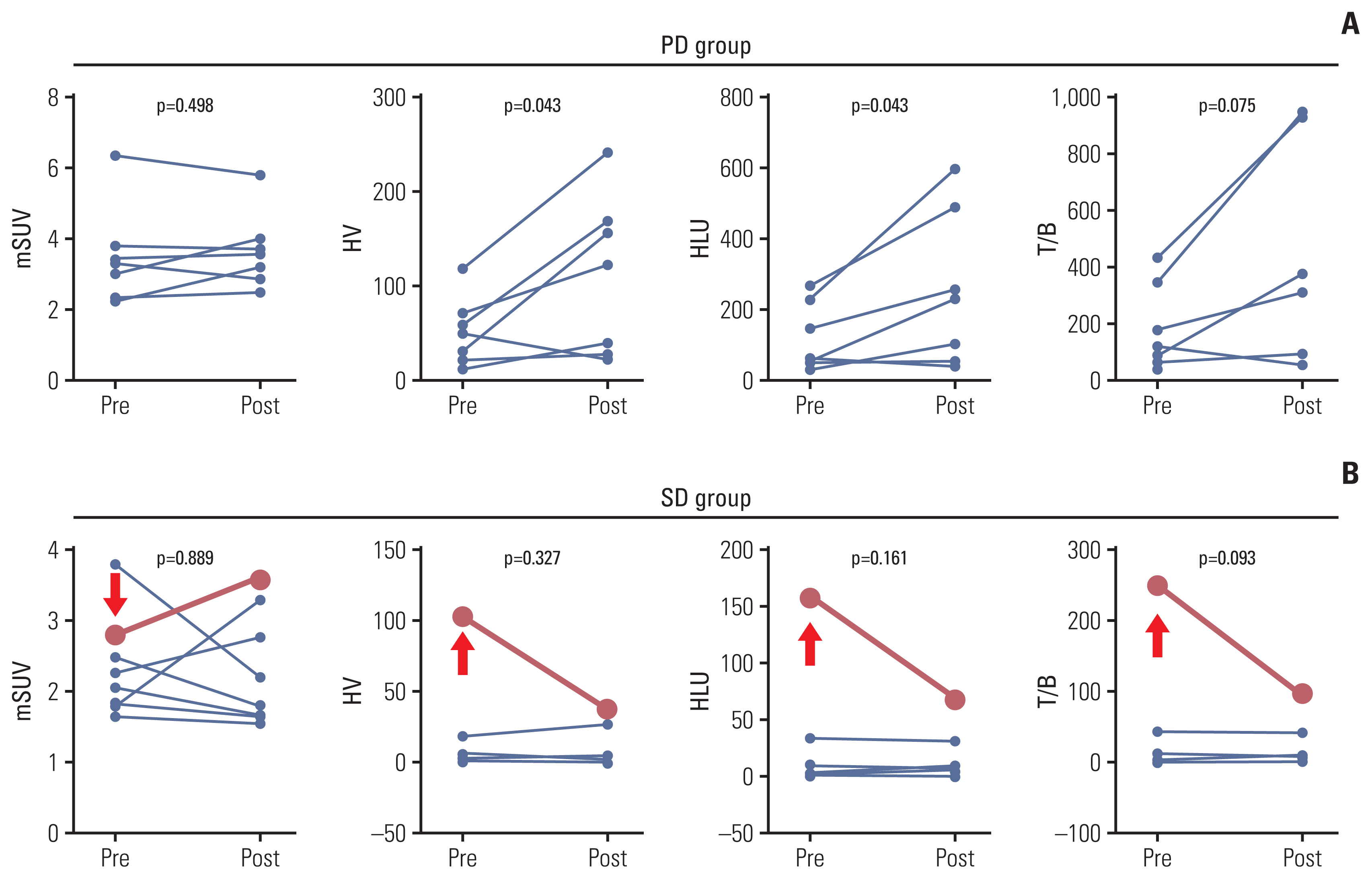
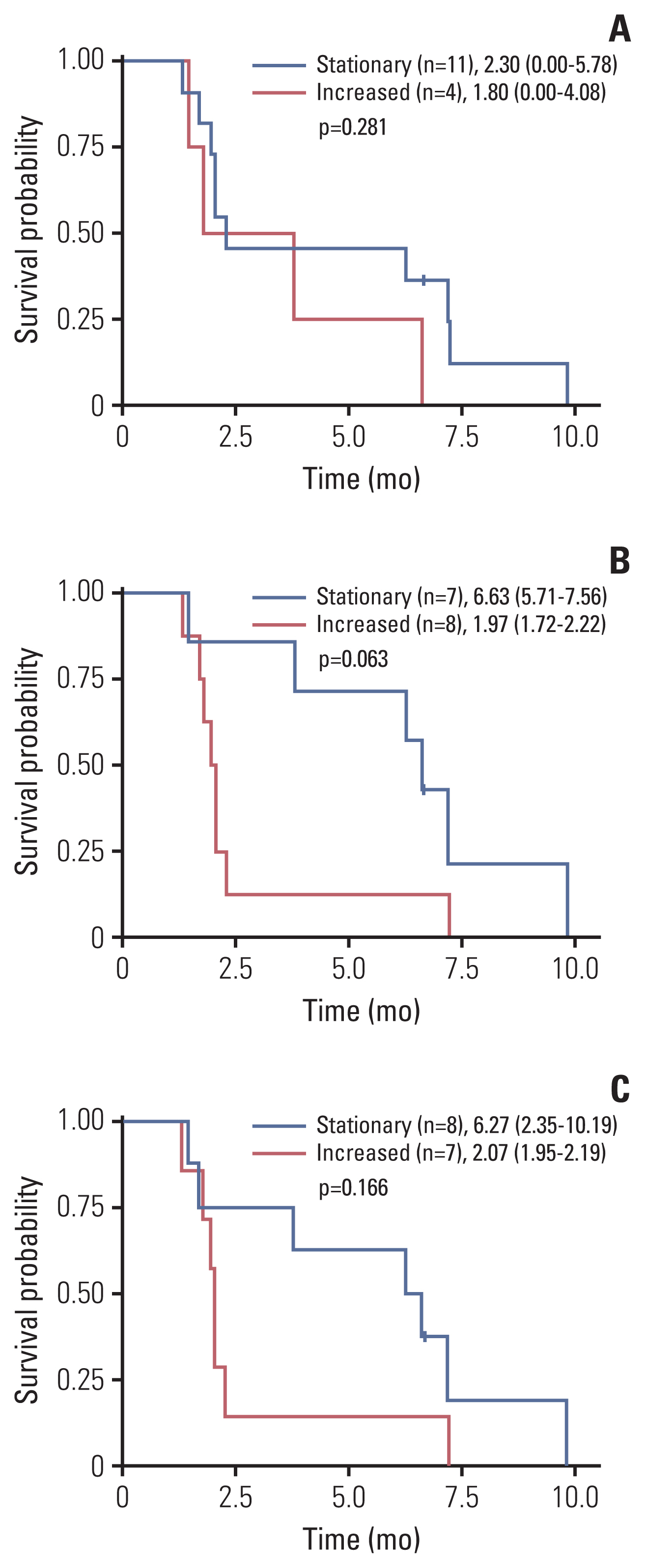
Twenty patients were treated with evofosfamide, with 16 response-evaluable patients. There was no objective response; stable disease was observed in nine patients, with a DCR of 56.25%. 4m-PFSR was 40.6%. Median PFS was 3.60 months (95% confidence interval [CI], 1.68 to 5.52). Median OS was 6.37 months (95% CI, 3.94 to 8.79). Reduction of tumor metabolic activity was observed in eight of 15 patients (53.3%). High baseline hypoxic parameters were associated with poor PFS. Change of hypoxic parameters between pretreatment and post-treatment reflected hypoxic-activated drug response. There was no treatment-related death.
Conclusion
Evofosfamide as second-line treatment of advanced BTC showed acceptable safety and comparable efficacy to other agents. Changes in volumetric parameters measured with FMISO PET, showing the degree of tumor hypoxia, reflected the response to evofosfamide based on the mode of action.
Keyword
Figure
Reference
-
References
1. Randi G, Franceschi S, La Vecchia C. Gallbladder cancer worldwide: geographical distribution and risk factors. Int J Cancer. 2006; 118:1591–602.
Article2. Marcano-Bonilla L, Mohamed EA, Mounajjed T, Roberts LR. Biliary tract cancers: epidemiology, molecular pathogenesis and genetic risk associations. Chin Clin Oncol. 2016; 5:61.
Article3. de Groen PC, Gores GJ, LaRusso NF, Gunderson LL, Nagorney DM. Biliary tract cancers. N Engl J Med. 1999; 341:1368–78.
Article4. Cereda S, Belli C, Rognone A, Mazza E, Reni M. Second-line therapy in advanced biliary tract cancer: what should be the standard? Crit Rev Oncol Hematol. 2013; 88:368–74.
Article5. Brieau B, Dahan L, De Rycke Y, Boussaha T, Vasseur P, Tougeron D, et al. Second-line chemotherapy for advanced biliary tract cancer after failure of the gemcitabine-platinum combination: a large multicenter study by the Association des Gastro-Enterologues Oncologues. Cancer. 2015; 121:3290–7.6. Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010; 362:1273–81.
Article7. Hockel M, Vaupel P. Tumor hypoxia: definitions and current clinical, biologic, and molecular aspects. J Natl Cancer Inst. 2001; 93:266–76.
Article8. Vanichapol T, Leelawat K, Hongeng S. Hypoxia enhances cholangiocarcinoma invasion through activation of hepatocyte growth factor receptor and the extracellular signalregulated kinase signaling pathway. Mol Med Rep. 2015; 12:3265–72.
Article9. Okumura Y, Noda T, Eguchi H, Sakamoto T, Iwagami Y, Yamada D, et al. Hypoxia-induced PLOD2 is a key regulator in epithelial-mesenchymal transition and chemoresistance in biliary tract cancer. Ann Surg Oncol. 2018; 25:3728–37.
Article10. Meng F, Evans JW, Bhupathi D, Banica M, Lan L, Lorente G, et al. Molecular and cellular pharmacology of the hypoxia-activated prodrug TH-302. Mol Cancer Ther. 2012; 11:740–51.
Article11. Liu Q, Sun JD, Wang J, Ahluwalia D, Baker AF, Cranmer LD, et al. TH-302, a hypoxia-activated prodrug with broad in vivo preclinical combination therapy efficacy: optimization of dosing regimens and schedules. Cancer Chemother Pharmacol. 2012; 69:1487–98.
Article12. Jung D, Jiao H, Duan JX, Matteucci M, Wang R. Metabolism and excretion of TH-302 in dogs. Xenobiotica. 2012; 42:687–700.
Article13. Tamaki N, Hirata K. Tumor hypoxia: a new PET imaging biomarker in clinical oncology. Int J Clin Oncol. 2016; 21:619–25.
Article14. Lopci E, Grassi I, Chiti A, Nanni C, Cicoria G, Toschi L, et al. PET radiopharmaceuticals for imaging of tumor hypoxia: a review of the evidence. Am J Nucl Med Mol Imaging. 2014; 4:365–84.15. Tachibana I, Nishimura Y, Hanaoka K, Inada M, Fukuda K, Tatebe H, et al. Tumor hypoxia detected by (18)F-fluoromisonidazole positron emission tomography (FMISO PET) as a prognostic indicator of radiotherapy (RT). Anticancer Res. 2018; 38:1775–81.
Article16. Szeto MD, Chakraborty G, Hadley J, Rockne R, Muzi M, Alvord EC Jr, et al. Quantitative metrics of net proliferation and invasion link biological aggressiveness assessed by MRI with hypoxia assessed by FMISO-PET in newly diagnosed glioblastomas. Cancer Res. 2009; 69:4502–9.
Article17. Yi JH, Thongprasert S, Lee J, Doval DC, Park SH, Park JO, et al. A phase II study of sunitinib as a second-line treatment in advanced biliary tract carcinoma: a multicentre, multinational study. Eur J Cancer. 2012; 48:196–201.
Article18. Lamarca A, Hubner RA, David Ryder W, Valle JW. Second-line chemotherapy in advanced biliary cancer: a systematic review. Ann Oncol. 2014; 25:2328–38.
Article19. Bekaert L, Valable S, Lechapt-Zalcman E, Ponte K, Collet S, Constans JM, et al. [18F]-FMISO PET study of hypoxia in gliomas before surgery: correlation with molecular markers of hypoxia and angiogenesis. Eur J Nucl Med Mol Imaging. 2017; 44:1383–92.
Article20. Hicks RJ, Rischin D, Fisher R, Binns D, Scott AM, Peters LJ. Utility of FMISO PET in advanced head and neck cancer treated with chemoradiation incorporating a hypoxia-targeting chemotherapy agent. Eur J Nucl Med Mol Imaging. 2005; 32:1384–91.
Article21. Sato J, Kitagawa Y, Yamazaki Y, Hata H, Asaka T, Miyakoshi M, et al. Advantage of FMISO-PET over FDG-PET for predicting histological response to preoperative chemotherapy in patients with oral squamous cell carcinoma. Eur J Nucl Med Mol Imaging. 2014; 41:2031–41.
Article22. Sachpekidis C, Thieke C, Askoxylakis V, Nicolay NH, Huber PE, Thomas M, et al. Combined use of (18)F-FDG and (18)F-FMISO in unresectable non-small cell lung cancer patients planned for radiotherapy: a dynamic PET/CT study. Am J Nucl Med Mol Imaging. 2015; 5:127–42.23. Thureau S, Dubray B, Modzelewski R, Bohn P, Hapdey S, Vincent S, et al. FDG and FMISO PET-guided dose escalation with intensity-modulated radiotherapy in lung cancer. Radiat Oncol. 2018; 13:208.
Article24. Peeters SG, Zegers CM, Lieuwes NG, van Elmpt W, Eriksson J, van Dongen GA, et al. A comparative study of the hypoxia PET tracers (18)F-HX4, (18)F-FAZA, and (18)F-FMISO in a preclinical tumor model. Int J Radiat Oncol Biol Phys. 2015; 91:351–9.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Kinetic Evaluation of the Hypoxia Radiotracers [ 18 F]FMISO and [ 18 F] FAZA in Dogs with Spontaneous Tumors Using Dynamic PET/CT Imaging
- Quantification of Hypoxia in Human Glioblastoma using PET with 18F-FMISO
- The Role of Hypoxia in Brain Tumor Immune Responses
- New Trend of tumor PET imaging radiopharmaceuticals
- The Role of Positron Emission Tomography(PET) in Radiation Treatment Planning