Umbilical Cord-Mesenchymal Stem Cell-Conditioned Medium Improves Insulin Resistance in C2C12 Cell
- Affiliations
-
- 1Department of Internal Medicine, CHA Bundang Medical Center, CHA University School of Medicine, Seongnam, Korea.
- 2Department of Biotechnology, CHA University, Seongnam, Korea.
- 3Department of Rehabilitation Medicine, CHA Bundang Medical Center, CHA University School of Medicine, Seongnam, Korea.
- KMID: 2514202
- DOI: http://doi.org/10.4093/dmj.2019.0191
Abstract
Background Umbilical cord-mesenchymal stem cell-conditioned medium (UC-MSC-CM) has emerged as a promising cell-free therapy. The aim of this study was to explore the therapeutic effects of UC-MSC-CM on insulin resistance in C2C12 cell.
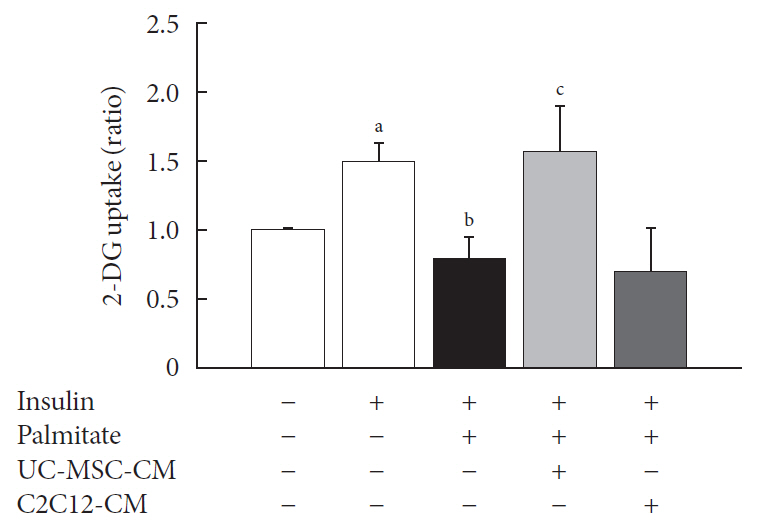
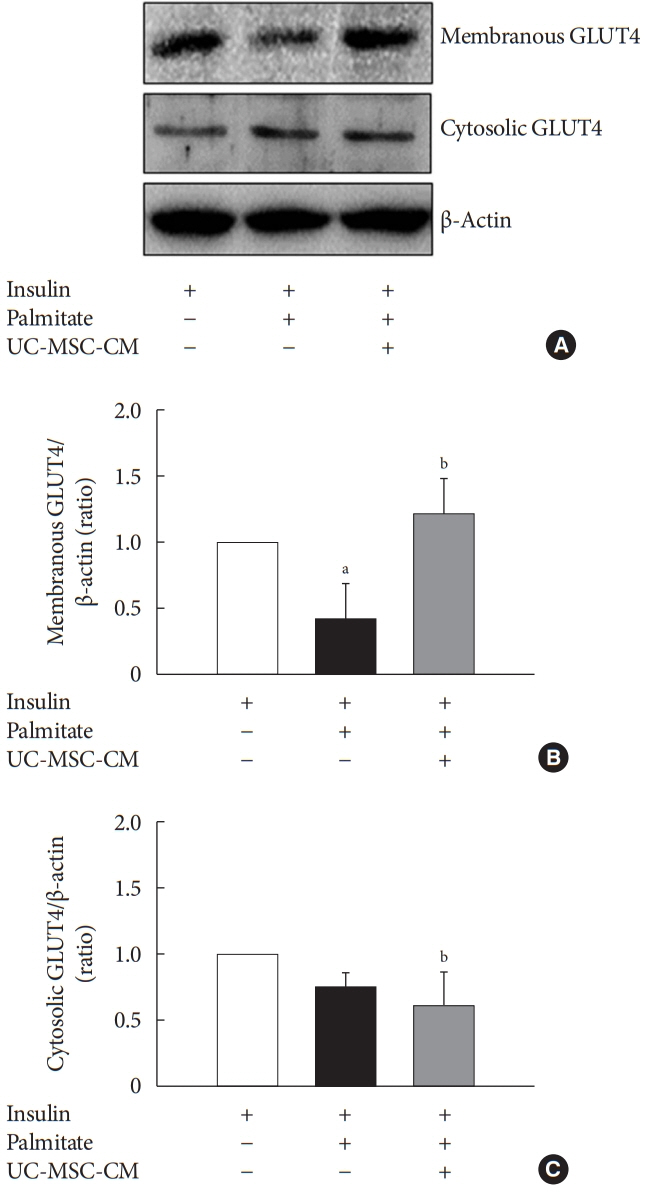
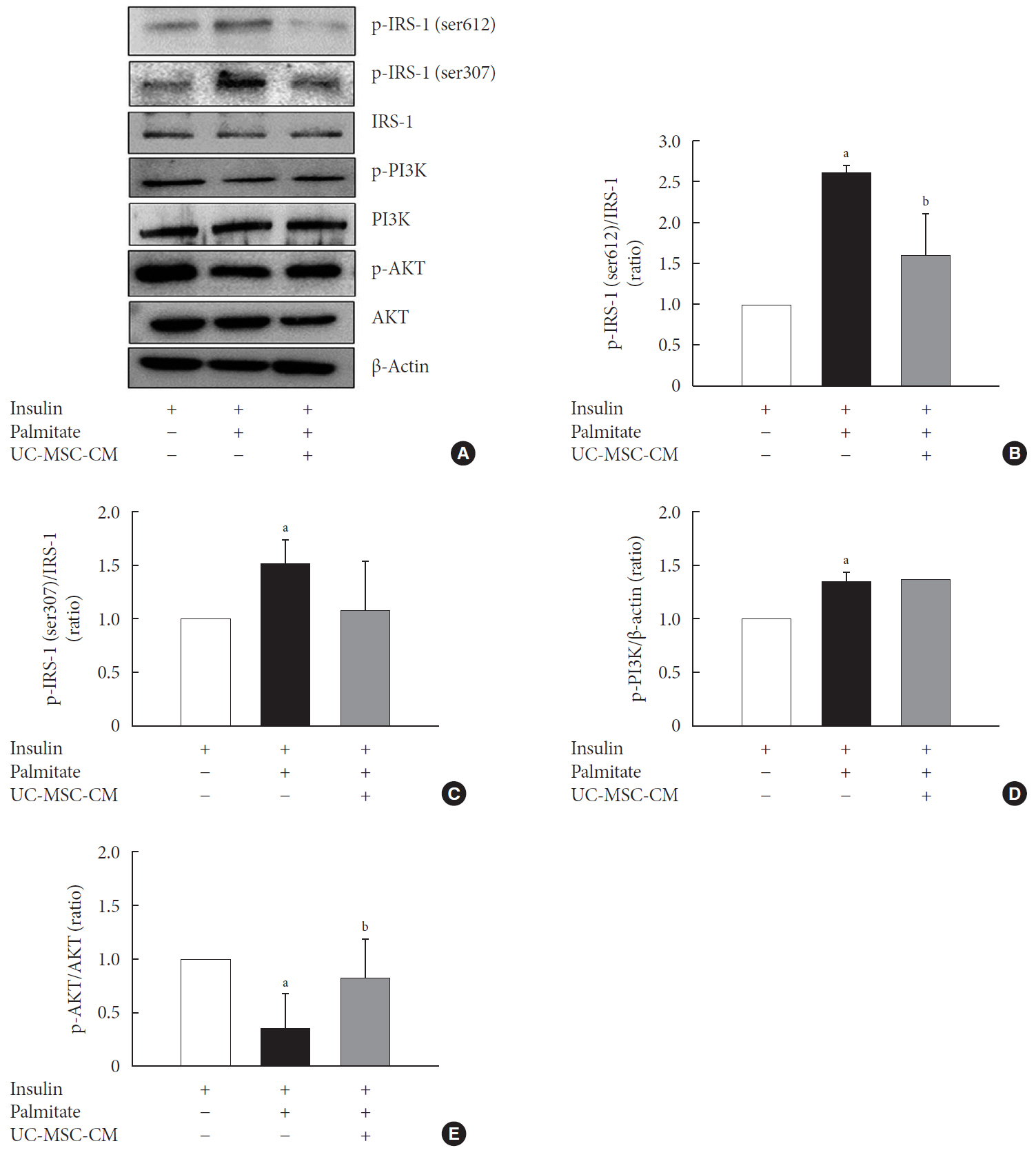
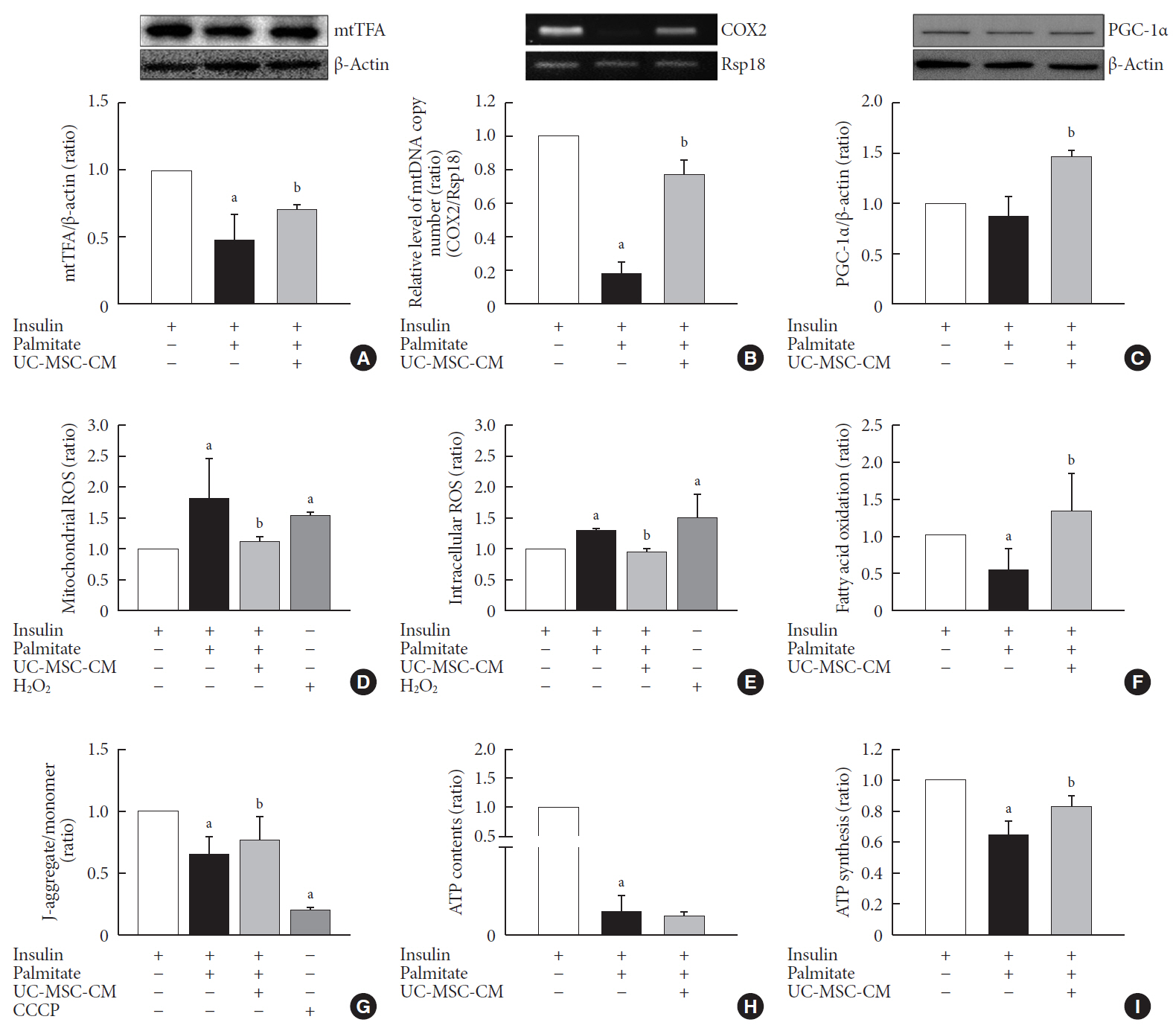
Methods Insulin resistance was induced by palmitate. Effects of UC-MSC-CM on insulin resistance were evaluated using glucose uptake, glucose transporter type 4 (GLUT4) translocation, the insulin-signaling pathway, and mitochondrial contents and functions in C2C12 cell.
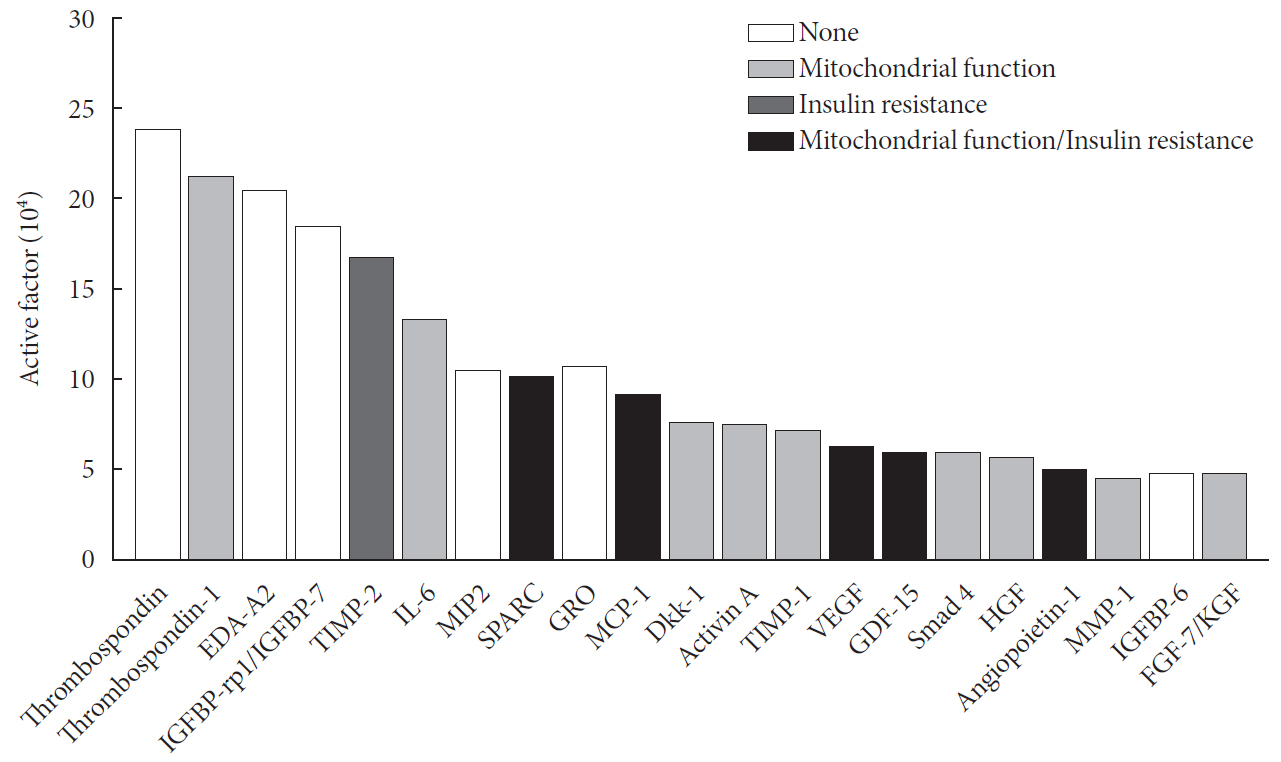
Results Glucose uptake was improved by UC-MSC-CM. UC-MSC-CM treatment increased only in membranous GLUT4 expression, not in cytosolic GLUT4 expression. It restored the insulin-signaling pathway in insulin receptor substrate 1 and protein kinase B. Mitochondrial contents evaluated by mitochondrial transcription factor A, mitochondrial DNA copy number, and peroxisome proliferator-activated receptor gamma coactivator 1-alpha were increased by UC-MSC-CM. In addition, UC-MSC-CM significantly decreased mitochondrial reactive oxygen species and increased fatty acid oxidation and mitochondrial membrane potential. There was no improvement in adenosine triphosphate (ATP) contents, but ATP synthesis was improved by UC-MSC-CM. Cytokine and active factor analysis of UC-MSC-CM showed that it contained many regulators inhibiting insulin resistance.
Conclusion UC-MSC-CM improves insulin resistance with multiple mechanisms in C2C12 cell.
Keyword
Figure
Reference
-
1. Ahn YH. A journey to understand glucose homeostasis: starting from rat glucose transporter type 2 promoter cloning to hyperglycemia. Diabetes Metab J. 2018; 42:465–471.
Article2. Kwak SH, Park KS. Pathophysiology of type 2 diabetes in Koreans. Endocrinol Metab (Seoul). 2018; 33:9–16.
Article3. Kim KS, Lee BW, Kim YJ, Lee DH, Cha BS, Park CY. Nonalcoholic fatty liver disease and diabetes. Part II: treatment. Diabetes Metab J. 2019; 43:127–143.
Article4. American Diabetes Association. 6. Glycemic targets: standards of medical care in diabetes. 2019. Diabetes Care. 2019; 42 Suppl 1:S61–S70.5. Ko SH, Han K, Lee YH, Noh J, Park CY, Kim DJ, et al. TaskForce Team for the Diabetes Fact Sheet of the Korean Diabetes Association. Past and current status of adult type 2 diabetes mellitus management in Korea: a National Health Insurance Service database analysis. Diabetes Metab J. 2018; 42:93–100.
Article6. Won JC, Lee JH, Kim JH, Kang ES, Won KC, Kim DJ, et al. Diabetes fact sheet in Korea, 2016: an appraisal of current status. Diabetes Metab J. 2018; 42:415–424.
Article7. Diecke S, Jung SM, Lee J, Ju JH. Recent technological updates and clinical applications of induced pluripotent stem cells. Korean J Intern Med. 2014; 29:547–557.
Article8. Zang L, Hao H, Liu J, Li Y, Han W, Mu Y. Mesenchymal stem cell therapy in type 2 diabetes mellitus. Diabetol Metab Syndr. 2017; 9:36.
Article9. Nagamura-Inoue T, He H. Umbilical cord-derived mesenchymal stem cells: their advantages and potential clinical utility. World J Stem Cells. 2014; 6:195–202.
Article10. Liu X, Zheng P, Wang X, Dai G, Cheng H, Zhang Z, et al. A preliminary evaluation of efficacy and safety of Wharton’s jelly mesenchymal stem cell transplantation in patients with type 2 diabetes mellitus. Stem Cell Res Ther. 2014; 5:57.
Article11. Sun X, Hao H, Han Q, Song X, Liu J, Dong L, et al. Human umbilical cord-derived mesenchymal stem cells ameliorate insulin resistance by suppressing NLRP3 inflammasome-mediated inflammation in type 2 diabetes rats. Stem Cell Res Ther. 2017; 8:241.
Article12. Ranganath SH, Levy O, Inamdar MS, Karp JM. Harnessing the mesenchymal stem cell secretome for the treatment of cardiovascular disease. Cell Stem Cell. 2012; 10:244–258.
Article13. Periasamy M, Herrera JL, Reis FCG. Skeletal muscle thermogenesis and its role in whole body energy metabolism. Diabetes Metab J. 2017; 41:327–336.
Article14. American Diabetes Association. 9. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes. 2019. Diabetes Care. 2019; 42 Suppl 1:S90–S102.15. Ko SH, Hur KY, Rhee SY, Kim NH, Moon MK, Park SO, et al. Committee of Clinical Practice Guideline of Korean Diabetes Association. Antihyperglycemic agent therapy for adult patients with type 2 diabetes mellitus 2017: a position statement of the Korean Diabetes Association. Diabetes Metab J. 2017; 41:337–348.
Article16. Raveendran AV, Deshpandae A, Joshi SR. Therapeutic role of yoga in type 2 diabetes. Endocrinol Metab (Seoul). 2018; 33:307–317.
Article17. Kim MJ, Kim ZH, Kim SM, Choi YS. Conditioned medium derived from umbilical cord mesenchymal stem cells regenerates atrophied muscles. Tissue Cell. 2016; 48:533–543.
Article18. Li HB, Yang YR, Mo ZJ, Ding Y, Jiang WJ. Silibinin improves palmitate-induced insulin resistance in C2C12 myotubes by attenuating IRS-1/PI3K/Akt pathway inhibition. Braz J Med Biol Res. 2015; 48:440–446.
Article19. Huang X, Liu G, Guo J, Su Z. The PI3K/AKT pathway in obesity and type 2 diabetes. Int J Biol Sci. 2018; 14:1483–1496.
Article20. Choi YS, Kim S, Pak YK. Mitochondrial transcription factor A (mtTFA) and diabetes. Diabetes Res Clin Pract. 2001; 54 Suppl 2:S3–S9.
Article21. Handschin C, Spiegelman BM. The role of exercise and PGC1alpha in inflammation and chronic disease. Nature. 2008; 454:463–469.22. Samjoo IA, Safdar A, Hamadeh MJ, Glover AW, Mocellin NJ, Santana J, et al. Markers of skeletal muscle mitochondrial function and lipid accumulation are moderately associated with the homeostasis model assessment index of insulin resistance in obese men. PLoS One. 2013; 8:e66322.
Article23. Yuan Y, Shi M, Li L, Liu J, Chen B, Chen Y, et al. Mesenchymal stem cell-conditioned media ameliorate diabetic endothelial dysfunction by improving mitochondrial bioenergetics via the Sirt1/AMPK/PGC-1α pathway. Clin Sci (Lond). 2016; 130:2181–2198.
Article24. Kim MJ, Hwang JW, Yun CK, Lee Y, Choi YS. Delivery of exogenous mitochondria via centrifugation enhances cellular metabolic function. Sci Rep. 2018; 8:3330.
Article25. Kadam S, Muthyala S, Nair P, Bhonde R. Human placenta-derived mesenchymal stem cells and islet-like cell clusters generated from these cells as a novel source for stem cell therapy in diabetes. Rev Diabet Stud. 2010; 7:168–182.
Article26. Xie Z, Hao H, Tong C, Cheng Y, Liu J, Pang Y, et al. Human umbilical cord-derived mesenchymal stem cells elicit macrophages into an anti-inflammatory phenotype to alleviate insulin resistance in type 2 diabetic rats. Stem Cells. 2016; 34:627–639.
Article27. Shree N, Bhonde RR. Conditioned media from adipose tissue derived mesenchymal stem cells reverse insulin resistance in cellular models. J Cell Biochem. 2017; 118:2037–2043.
Article28. Park CM, Kim MJ, Kim SM, Park JH, Kim ZH, Choi YS. Umbilical cord mesenchymal stem cell-conditioned media prevent muscle atrophy by suppressing muscle atrophy-related proteins and ROS generation. In Vitro Cell Dev Biol Anim. 2016; 52:68–76.
Article29. Yi HS. Implications of mitochondrial unfolded protein response and mitokines: a perspective on fatty liver diseases. Endocrinol Metab (Seoul). 2019; 34:39–46.
Article30. Morino K, Petersen KF, Shulman GI. Molecular mechanisms of insulin resistance in humans and their potential links with mitochondrial dysfunction. Diabetes. 2006; 55 Suppl 2:S9–S15.
Article31. Pagel-Langenickel I, Bao J, Pang L, Sack MN. The role of mitochondria in the pathophysiology of skeletal muscle insulin resistance. Endocr Rev. 2010; 31:25–51.
Article32. Szendroedi J, Phielix E, Roden M. The role of mitochondria in insulin resistance and type 2 diabetes mellitus. Nat Rev Endocrinol. 2011; 8:92–103.
Article33. Fealy CE, Mulya A, Axelrod CL, Kirwan JP. Mitochondrial dynamics in skeletal muscle insulin resistance and type 2 diabetes. Transl Res. 2018; 202:69–82.
Article34. Jaworski DM, Sideleva O, Stradecki HM, Langlois GD, Habibovic A, Satish B, et al. Sexually dimorphic diet-induced insulin resistance in obese tissue inhibitor of metalloproteinase-2 (TIMP-2)-deficient mice. Endocrinology. 2011; 152:1300–1313.
Article35. Xu L, Ping F, Yin J, Xiao X, Xiang H, Ballantyne CM, et al. Elevated plasma SPARC levels are associated with insulin resistance, dyslipidemia, and inflammation in gestational diabetes mellitus. PLoS One. 2013; 8:e81615.
Article36. Kempf T, Guba-Quint A, Torgerson J, Magnone MC, Haefliger C, Bobadilla M, et al. Growth differentiation factor 15 predicts future insulin resistance and impaired glucose control in obese nondiabetic individuals: results from the XENDOS trial. Eur J Endocrinol. 2012; 167:671–678.
Article37. Frazier EP, Isenberg JS, Shiva S, Zhao L, Schlesinger P, Dimitry J, et al. Age-dependent regulation of skeletal muscle mitochondria by the thrombospondin-1 receptor CD47. Matrix Biol. 2011; 30:154–161.
Article38. Sandhir R, Halder A, Sunkaria A. Mitochondria as a centrally positioned hub in the innate immune response. Biochim Biophys Acta Mol Basis Dis. 2017; 1863:1090–1097.
Article39. Wu L, Tan X, Liang L, Yu H, Wang C, Zhang D, et al. The role of mitochondria-associated reactive oxygen species in the amyloid β induced production of angiogenic factors by ARPE-19 cells. Curr Mol Med. 2017; 17:140–148.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Efficacy of a Hair Tonic Containing Human Umbilical Cord Blood Mesenchymal Stem Cell-derived Conditioned Media in Patients with Androgenetic Alopecia
- Effect of Conditioned Medium from Human Umbilical Cord-Derived Mesenchymal Stromal Cells on Rejuvenation of Nucleus Pulposus Derived Stem/Progenitor Cells from Degenerated Intervertebral Disc
- Endothelial progenitor cells and mesenchymal stem cells from human cord blood
- Enhanced Anti-Cancer Effects of Conditioned Medium from Hypoxic Human Umbilical Cord–Derived Mesenchymal Stem Cells
- Differentiation of Osteoblast Progenitor Cells from Human Umbilical Cord Blood