Age at Diagnosis and the Risk of Diabetic Nephropathy in Young Patients with Type 1 Diabetes Mellitus
- Affiliations
-
- 1Department of Internal Medicine, Gyeongsang National University Changwon Hospital, Gyeongsang National University College of Medicine, Changwon, Korea.
- 2Department of Internal Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 3Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea.
- 4Department of Internal Medicine, Gyeongsang National University Hospital, Gyeongsang National University College of Medicine, Jinju, Korea.
- 5Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- KMID: 2514073
- DOI: http://doi.org/10.4093/dmj.2019.0134
Abstract
Background The aim of this study was to evaluate characteristics and risk of diabetic complications according to age at diagnosis among young adults with type 1 diabetes mellitus (T1DM).
Methods A total of 255 T1DM patients aged less than 40 years were included. Patients were categorized into three groups (<20, 20 to 29, and 30 to 40 years) according to age at diagnosis. Diabetic nephropathy (DN) was defined when spot urine-albumin creatinine ratio was 300 mg/g or more and/or estimated glomerular filtration ratio (eGFR) level was 60 mL/min/1.73 m2 or less.
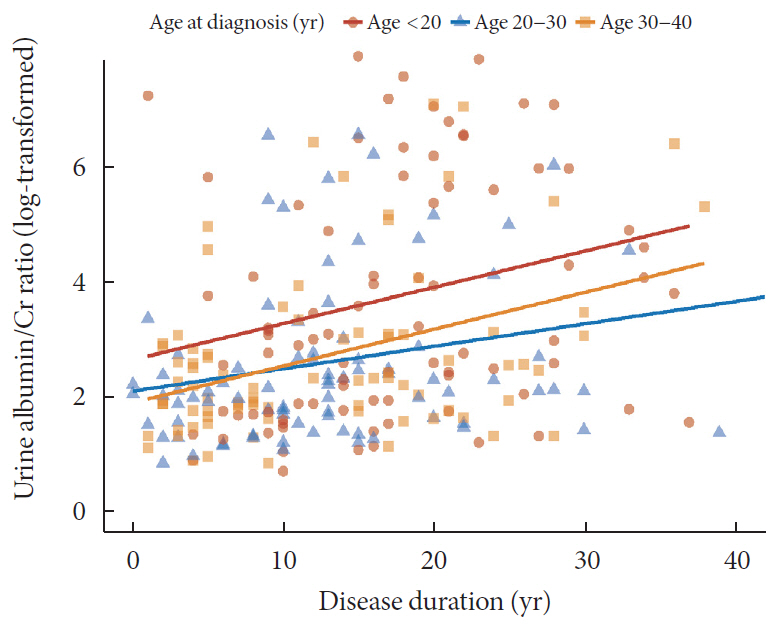
Results Median age at diagnosis was 25 years and disease duration was 14 years. Individuals diagnosed with T1DM at childhood/adolescent (age <20 years) had lower stimulated C-peptide levels. They received more intensive insulin treatment with higher total daily insulin doses compared to older onset groups. The prevalence of DN was higher in the childhood/adolescent-onset group than in older onset groups (25.3% vs. 15.3% vs. 9.6%,
P =0.022). The eGFR was inversely associated with disease duration whilst the degree of decrease was more prominent in the childhood/adolescent-onset group than in the later onset group (aged 30 to 40 years;P <0.001). Childhood/adolescent-onset group was independently associated with the risk of DN compared to the older onset group (aged 30 to 40 years; odds ratio, 3.47; 95% confidence interval, 1.45 to 8.33;P =0.005).Conclusion In individuals with childhood/adolescent-onset T1DM, the reduction in renal function is more prominent with disease duration. Early age-onset T1DM is an independent risk of DN.
Figure
Cited by 2 articles
-
Age at Diagnosis and the Risk of Diabetic Nephropathy in Young Patients with Type 1 Diabetes Mellitus (
Diabetes Metab J 2021;45:46-54)
Jong Ha Baek, Jae Hyeon Kim
Diabetes Metab J. 2021;45(2):281-282. doi: 10.4093/dmj.2021.0040.Age at Diagnosis and the Risk of Diabetic Nephropathy in Young Patients with Type 1 Diabetes Mellitus (
Diabetes Metab J 2021;45:46-54)
Ye Seul Yang, Tae Seo Sohn
Diabetes Metab J. 2021;45(2):277-278. doi: 10.4093/dmj.2021.0028.
Reference
-
1. Iqbal A, Novodvorsky P, Heller SR. Recent updates on type 1 diabetes mellitus management for clinicians. Diabetes Metab J. 2018; 42:3–18.
Article2. Tancredi M, Rosengren A, Svensson AM, Kosiborod M, Pivodic A, Gudbjornsdottir S, et al. Excess mortality among persons with type 2 diabetes. N Engl J Med. 2015; 373:1720–1732.
Article3. Secrest AM, Becker DJ, Kelsey SF, Laporte RE, Orchard TJ. Cause-specific mortality trends in a large population-based cohort with long-standing childhood-onset type 1 diabetes. Diabetes. 2010; 59:3216–3222.
Article4. Lee YB, Han K, Kim B, Jun JE, Lee SE, Ahn J, et al. Risk of end-stage renal disease from chronic kidney disease defined by decreased glomerular filtration rate in type 1 diabetes: a comparison with type 2 diabetes and the effect of metabolic syndrome. Diabetes Metab Res Rev. 2019; 35:e3197.
Article5. Orchard TJ, Secrest AM, Miller RG, Costacou T. In the absence of renal disease, 20 year mortality risk in type 1 diabetes is comparable to that of the general population: a report from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetologia. 2010; 53:2312–2319.6. Groop PH, Thomas MC, Moran JL, Waden J, Thorn LM, Makinen VP, et al. FinnDiane Study Group. The presence and severity of chronic kidney disease predicts all-cause mortality in type 1 diabetes. Diabetes. 2009; 58:1651–1658.
Article7. Writing Team for the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. Sustained effect of intensive treatment of type 1 diabetes mellitus on development and progression of diabetic nephropathy: the Epidemiology of Diabetes Interventions and Complications (EDIC) study. JAMA. 2003; 290:2159–2167.8. Jackson CE, Solomon SD, Gerstein HC, Zetterstrand S, Olofsson B, Michelson EL, et al. CHARM Investigators and Committees. Albuminuria in chronic heart failure: prevalence and prognostic importance. Lancet. 2009; 374:543–550.
Article9. Soedamah-Muthu SS, Chaturvedi N, Toeller M, Ferriss B, Reboldi P, Michel G, et al. EURODIAB Prospective Complications Study Group. Risk factors for coronary heart disease in type 1 diabetic patients in Europe: the EURODIAB Prospective Complications Study. Diabetes Care. 2004; 27:530–537.
Article10. Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, et al. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005; 353:2643–2653.
Article11. Zgibor JC, Ruppert K, Orchard TJ, Soedamah-Muthu SS, Fuller J, Chaturvedi N, et al. Development of a coronary heart disease risk prediction model for type 1 diabetes: the Pittsburgh CHD in Type 1 Diabetes Risk Model. Diabetes Res Clin Pract. 2010; 88:314–321.
Article12. Leslie RD, Kolb H, Schloot NC, Buzzetti R, Mauricio D, De Leiva A, et al. Diabetes classification: grey zones, sound and smoke: action LADA 1. Diabetes Metab Res Rev. 2008; 24:511–519.
Article13. Howson JM, Rosinger S, Smyth DJ, Boehm BO, Todd JA. ADBW-END Study Group. Genetic analysis of adult-onset autoimmune diabetes. Diabetes. 2011; 60:2645–2653.
Article14. Lee SA, Lee WJ, Kim EH, Yu JH, Jung CH, Koh EH, et al. Progression to insulin deficiency in Korean patients with type 2 diabetes mellitus positive for anti-GAD antibody. Diabet Med. 2011; 28:319–324.15. Lee YB, Han K, Kim B, Jin SM, Lee SE, Jun JE, et al. High proportion of adult cases and prevalence of metabolic syndrome in type 1 diabetes mellitus population in Korea: a nationwide study. Diabetes Metab J. 2019; 43:76–89.
Article16. Alleyn CR, Volkening LK, Wolfson J, Rodriguez-Ventura A, Wood JR, Laffel LM. Occurrence of microalbuminuria in young people with type 1 diabetes: importance of age and diabetes duration. Diabet Med. 2010; 27:532–537.
Article17. Asao K, Sarti C, Forsen T, Hyttinen V, Nishimura R, Matsushima M, et al. Diabetes Epidemiology Research International Mortality Study Group. Long-term mortality in nationwide cohorts of childhood-onset type 1 diabetes in Japan and Finland. Diabetes Care. 2003; 26:2037–2042.
Article18. Jin SM, Baek JH, Suh S, Jung CH, Lee WJ, Park CY, et al. Factors associated with greater benefit of a national reimbursement policy for blood glucose test strips in adult patients with type 1 diabetes: a prospective cohort study. J Diabetes Investig. 2017; 9:549–557.
Article19. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009; 150:604–612.
Article20. Yoon HJ, Lee YH, Kim KJ, Kim SR, Kang ES, Cha BS, et al. Glycated albumin levels in patients with type 2 diabetes increase relative to HbA1c with time. Biomed Res Int. 2015; 2015:576306.21. Kuhtreiber WM, Washer SL, Hsu E, Zhao M, Reinhold P 3rd, Burger D, et al. Low levels of C-peptide have clinical significance for established type 1 diabetes. Diabet Med. 2015; 32:1346–1353.
Article22. The DCCT Research Group. Effects of age, duration and treatment of insulin-dependent diabetes mellitus on residual beta-cell function: observations during eligibility testing for the Diabetes Control and Complications Trial (DCCT). J Clin Endocrinol Metab. 1987; 65:30–36.23. Steffes MW, Sibley S, Jackson M, Thomas W. Beta-cell function and the development of diabetes-related complications in the diabetes control and complications trial. Diabetes Care. 2003; 26:832–836.24. Lachin JM, Bebu I, Bergenstal RM, Pop-Busui R, Service FJ, Zinman B, et al. DCCT/EDIC Research Group. Association of glycemic variability in type 1 diabetes with progression of microvascular outcomes in the diabetes control and complications trial. Diabetes Care. 2017; 40:777–783.
Article25. Kilpatrick ES, Rigby AS, Atkin SL. A1C variability and the risk of microvascular complications in type 1 diabetes: data from the Diabetes Control and Complications Trial. Diabetes Care. 2008; 31:2198–2202.
Article26. Kilpatrick ES, Rigby AS, Atkin SL. The effect of glucose variability on the risk of microvascular complications in type 1 diabetes. Diabetes Care. 2006; 29:1486–1490.
Article27. Gorst C, Kwok CS, Aslam S, Buchan I, Kontopantelis E, Myint PK, et al. Long-term glycemic variability and risk of adverse outcomes: a systematic review and meta-analysis. Diabetes Care. 2015; 38:2354–2369.
Article28. Rawshani A, Sattar N, Franzen S, Rawshani A, Hattersley AT, Svensson AM, et al. Excess mortality and cardiovascular disease in young adults with type 1 diabetes in relation to age at onset: a nationwide, register-based cohort study. Lancet. 2018; 392:477–486.
Article29. Harjutsalo V, Maric-Bilkan C, Forsblom C, Groop PH. Impact of sex and age at onset of diabetes on mortality from ischemic heart disease in patients with type 1 diabetes. Diabetes Care. 2014; 37:144–148.
Article30. Mollsten A, Svensson M, Waernbaum I, Berhan Y, Schon S, Nystrom L, et al. Swedish Childhood Diabetes Study Group. Diabetes Incidence Study in Sweden. Swedish Renal Registry. Cumulative risk, age at onset, and sex-specific differences for developing end-stage renal disease in young patients with type 1 diabetes: a nationwide population-based cohort study. Diabetes. 2010; 59:1803–1808.
Article31. Svensson M, Nystrom L, Schon S, Dahlquist G. Age at onset of childhood-onset type 1 diabetes and the development of end-stage renal disease: a nationwide population-based study. Diabetes Care. 2006; 29:538–542.
Article32. Helve J, Sund R, Arffman M, Harjutsalo V, Groop PH, Gronhagen-Riska C, et al. Incidence of end-stage renal disease in patients with type 1 diabetes. Diabetes Care. 2018; 41:434–439.
Article33. Forga L, Goni MJ, Ibanez B, Cambra K, Garcia-Mouriz M, Iriarte A. Influence of age at diagnosis and time-dependent risk factors on the development of diabetic retinopathy in patients with type 1 diabetes. J Diabetes Res. 2016; 2016:9898309.
Article34. Kullberg CE, Abrahamsson M, Arnqvist HJ, Finnstrom K, Ludvigsson J. VISS Study Group. Prevalence of retinopathy differs with age at onset of diabetes in a population of patients with type 1 diabetes. Diabet Med. 2002; 19:924–931.
Article35. Cho YH, Craig ME, Donaghue KC. Puberty as an accelerator for diabetes complications. Pediatr Diabetes. 2014; 15:18–26.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Diabetic Nephropathy in Childhood and Adolescence (I) : Clinical Features
- Age at Diagnosis and the Risk of Diabetic Nephropathy in Young Patients with Type 1 Diabetes Mellitus (Diabetes Metab J 2021;45:46-54)
- A Case of Diabetic Nephropathy Progressed to End-Stage Renal Disease in an Adolescent with Type 1 Diabetes
- Epidemiology and Clinical Course of Diabetic Nephropathy; Is There Any Differences in Prevalence and Incidence of Diabetic Nephropathy between Type 1 and Type 2 Diabetes Mellitus?
- Glycemic Control in Diabetic Patients with Diabetic Nephropathy