Blood Res.
2021 Mar;56(1):31-37. 10.5045/br.2021.2020260.
Real-world management of chronic myeloid leukemia in South Korea: the TARGET survey
- Affiliations
-
- 1Division of Hematology Oncology, Department of Internal Medicine, Hanyang University Hospital, Hanyang University College of Medicine, Korea.
- 2Division of Hematology Oncology, Department of Internal Medicine, Soonchunhyang University College of Medicine, Seoul, Korea.
- 3Division of Tumor Immunology and Center for Hematologic Malignancies, Research Institute and Hospital, National Cancer Center, Goyang, Korea.
- 4Division of Hematology, Gachon University Gil Medical Center, Gachon University College of Medicine, Incheon, Korea.
- 5Novartis, Ltd., Seoul, Korea.
- 6Novartis Korea, Seoul, Korea.
- 7Division of Hematology-Oncology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- KMID: 2514062
- DOI: http://doi.org/10.5045/br.2021.2020260
Abstract
- Background
The global TARGET survey examined real-world management of chronic myeloid leukemia (CML) compared with international guideline recommendations. This report focused on the responses of physicians from South Korea compared with those of physicians from the rest of the world (ROW).
Methods
The self-administered, online survey, comprising 23 questions and clinical case scenarios, was completed between April and August 2017. It was designed to gather information on practicing physicians and local practices for CML diagnosis, disease monitoring, treatment, and adverse event (AE) management.
Results
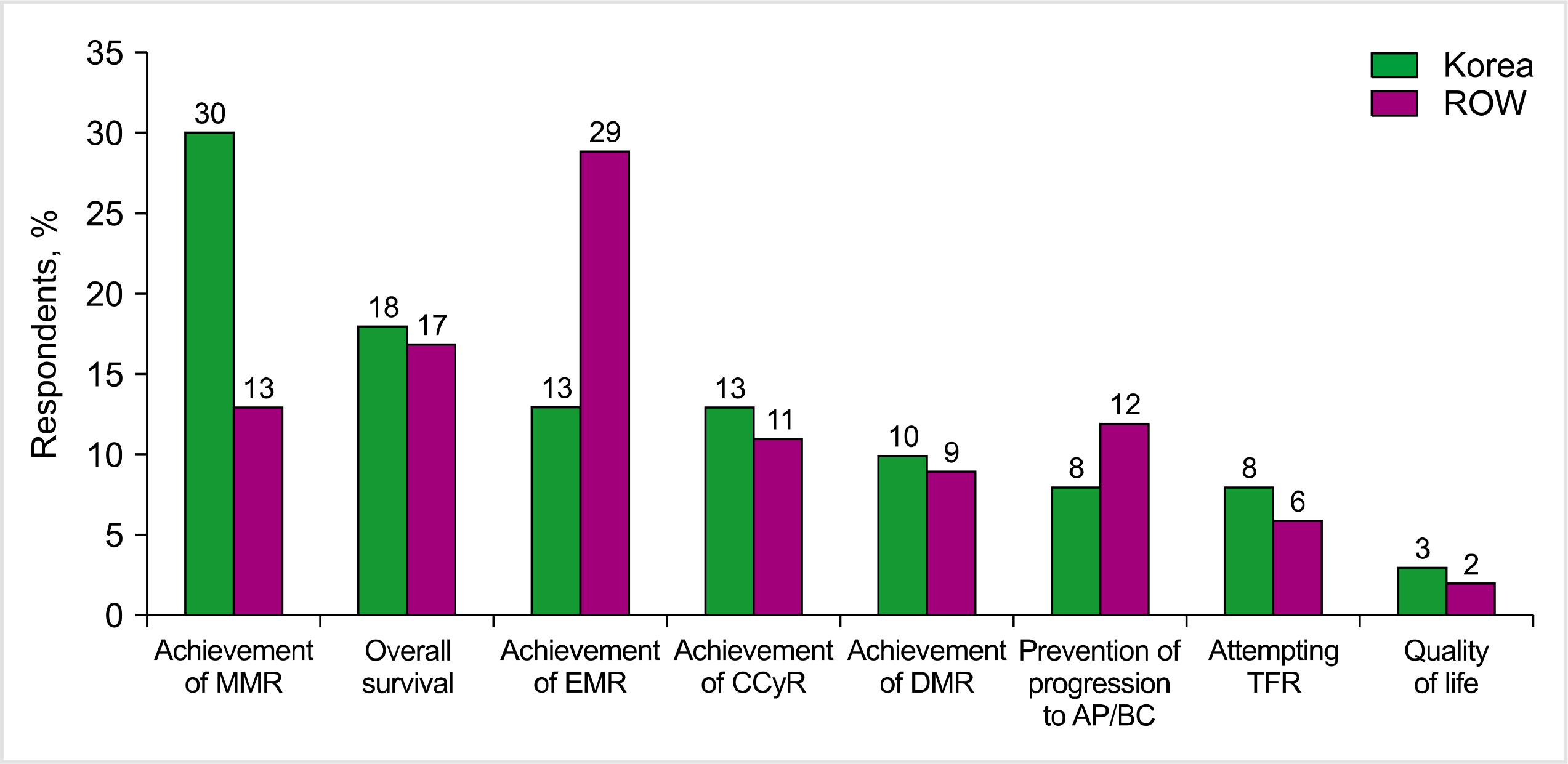
While there were similarities in the mutation analysis and treatment efficacy between Korea and the ROW, there were also differences in CML management. Initial diagnostic testing was more comprehensive in Korea than in the ROW, and there was significantly better access to standardized polymerase chain reaction testing. Assessment of BCR-ABL levels during the first 12 months of treatment was excellent in Korea, and there was greater frontline use of second-generation BCR-ABL tyrosine kinase inhibitors. Korean physicians were significantly less likely to switch therapy for hematologic AEs. Treatment-free remission was not an important goal of therapy among Korean or ROW physicians.
Conclusion
This study identified some differences in the current CML management between Korea and the ROW; CML management in Korean patients was generally in line with the current guidelines.
Keyword
Figure
Reference
-
1. O'Brien S, Radich JP, Abboud CN, et al. 2014; Chronic myelogenous leukemia, version 1.2015. J Natl Compr Canc Netw. 12:1590–610. DOI: 10.6004/jnccn.2014.0159. PMID: 25361806.2. Hochhaus A, Saussele S, Rosti G, et al. 2017; Chronic myeloid leukaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 28(Suppl 4):iv41–51. DOI: 10.1093/annonc/mdx219. PMID: 28881915.
Article3. Holyoake TL, Vetrie D. 2017; The chronic myeloid leukemia stem cell: stemming the tide of persistence. Blood. 129:1595–606. DOI: 10.1182/blood-2016-09-696013. PMID: 28159740.
Article4. Park EH, Lee H, Won YJ, et al. 2015; Nationwide statistical analysis of myeloid malignancies in Korea: incidence and survival rate from 1999 to 2012. Blood Res. 50:204–17. DOI: 10.5045/br.2015.50.4.204. PMID: 26770948. PMCID: PMC4705046.5. National Comprehensive Cancer Network. 2018. NCCN Practice Guidelines in Oncology: chronic myeloid leukemia. Version 4. National Comprehensive Cancer Network;Plymouth Meeting, PA: at https://www.nccn.org/professionals/physician_gls/pdf/cml.pdf. Accessed March 29, 2018.6. Kim DY, Lee JO, Kim KH, et al. 2015; Korean guidelines for treating chronic myelogenous leukemia-The Korean Society of Hematology Chronic Myelogenous Leukemia Working Party. Korean J Med. 88:406–19. DOI: 10.3904/kjm.2015.88.4.406.7. Baccarani M, Deininger MW, Rosti G, et al. 2013; European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 122:872–84. DOI: 10.1182/blood-2013-05-501569. PMID: 23803709. PMCID: PMC4915804.8. Turkina A, Wang J, Mathews V, et al. 2020; TARGET: a survey of real-world management of chronic myeloid leukaemia across 33 countries. Br J Haematol. 190:869–76. DOI: 10.1111/bjh.16599. PMID: 32227648.9. Gale RP, Hochhaus A. 2018; Therapy-free remission in chronic myeloid leukemia: possible mechanism. Expert Rev Hematol. 11:269–72. DOI: 10.1080/17474086.2018.1442213. PMID: 29448857.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Leukemia Cutis in Myelodysplastic Syndrome Evolving into An Atypical Chronic Myeloid Leukemia
- Acute myeloid leukemia arising from chronic myelomonocytic leukemia during hypomethylating therapy
- A case of chronic myeloid leukemia with features of essential thrombocythemia in peripheral blood and bone marrow
- A Case of Acute Myeloid Leukemia Concurrent With Untreated Chronic Lymphocytic Leukemia
- Classification of acute myeloid leukemia