J Korean Med Sci.
2021 Mar;36(11):e75. 10.3346/jkms.2021.36.e75.
Right Ventricular Longitudinal Conduction Delay in Patients with Brugada Syndrome
- Affiliations
-
- 1Heart Center of Chonnam National University Hospital, Gwangju, Korea
- 2Division of Cardiology, Department of Internal Medicine, Chonnam National University Medical School, Gwangju, Korea
- KMID: 2514051
- DOI: http://doi.org/10.3346/jkms.2021.36.e75
Abstract
- Background
The mechanism of Brugada syndrome (BrS) is still unclear, with different researchers favoring either the repolarization or depolarization hypothesis. Prolonged longitudinal activation time has been verified in only a small number of human right ventricles (RVs). The purpose of the present study was to demonstrate RV conduction delays in BrS.
Methods
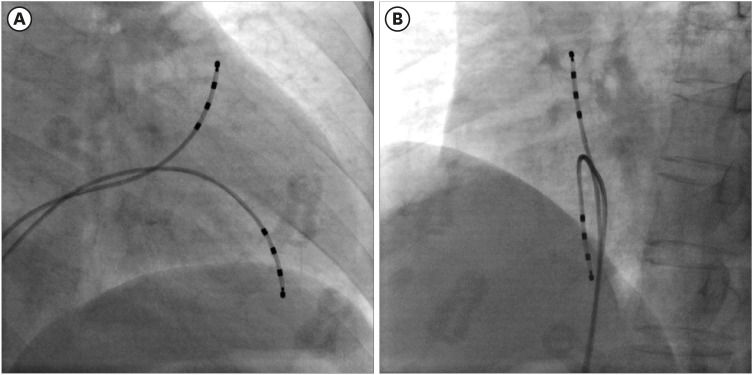
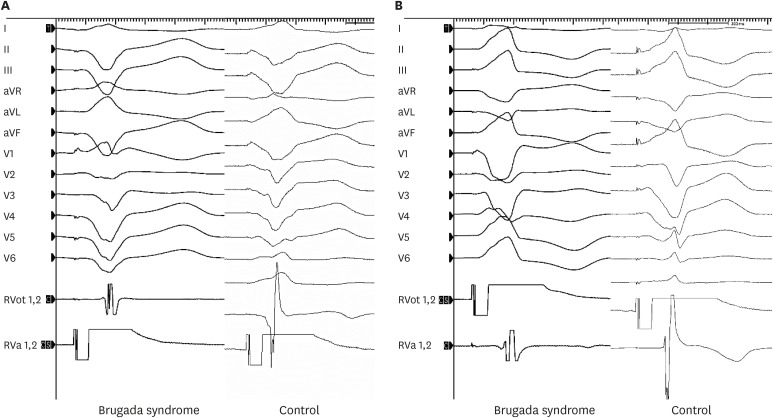
The RV outflow tract (RVOT)-to-RV apex (RVA) and RVA-to-RVOT conduction times were measured by endocardial stimulation and mapping in 7 patients with BrS and 14 controls.
Results
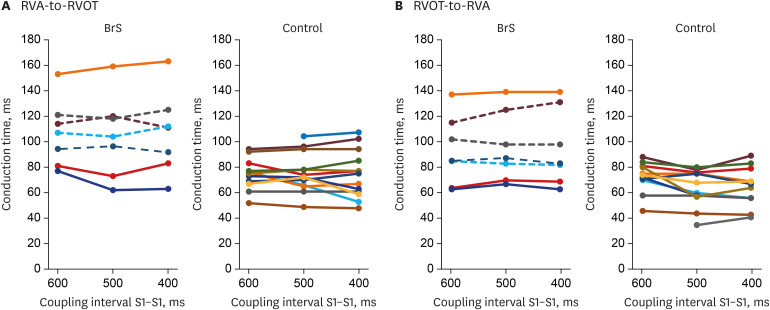
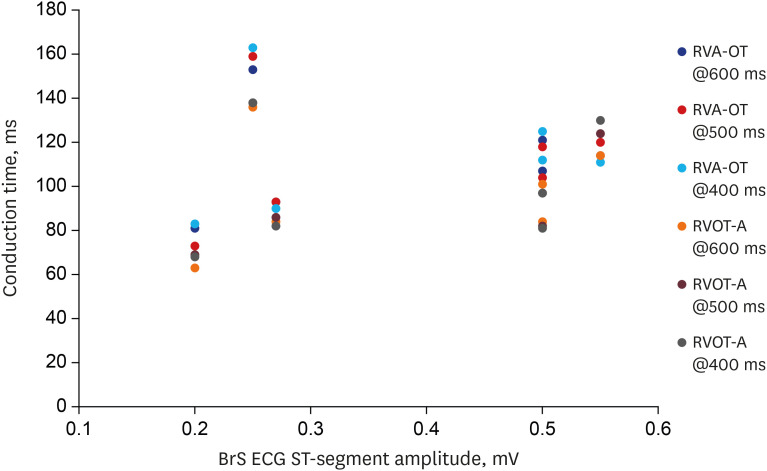
Patients with BrS had a longer PR interval (180 ± 12.6 vs. 142 ± 6.7 ms, P = 0.016). The RVA-to-RVOT conduction time was longer in the patients with BrS than in controls (stimulation at 600 ms, 107 ± 9.9 vs. 73 ± 3.4 ms, P= 0.001; stimulation at 500 ms, 104 ± 12.3 vs. 74 ± 4.2 ms, P = 0.037; stimulation at 400 ms, 107 ±12.2 vs. 73 ± 5.1 ms, P= 0.014). The RVOT-to-RVA conduction time was longer in the patients with BrS than in controls (stimulation at 500 ms, 95 ± 10.3 vs. 62 ± 4.1 ms, P= 0.007; stimulation at 400 ms, 94 ±11.2 vs. 64 ± 4.6 ms, P= 0.027). The difference in longitudinal conduction time was not significant when isoproterenol was administered.
Conclusion
The patients with BrS showed an RV longitudinal conduction delay obviously. These findings suggest that RV conduction delay might contribute to generate the BrS phenotype.
Keyword
Figure
Cited by 1 articles
-
Antiarrhythmic Effect of Artemisinin in an Ex-vivo Model of Brugada Syndrome Induced by NS5806
Hyung Ki Jeong, Seo Na Hong, Namsik Yoon, Ki Hong Lee, Hyung Wook Park, Jeong Gwan Cho
Korean Circ J. 2023;53(4):239-250. doi: 10.4070/kcj.2022.0312.
Reference
-
1. Van Malderen SCH, Daneels D, Kerkhove D, Peeters U, Theuns DAMJ, Droogmans S, et al. Prolonged right ventricular ejection delay in Brugada syndrome depends on the type of SCN5A variant - electromechanical coupling through tissue velocity imaging as a bridge between genotyping and phenotyping. Circ J. 2017; 82(1):53–61. PMID: 28781330.2. Postema PG, van Dessel PF, de Bakker JM, Dekker LR, Linnenbank AC, Hoogendijk MG, et al. Slow and discontinuous conduction conspire in Brugada syndrome: a right ventricular mapping and stimulation study. Circ Arrhythm Electrophysiol. 2008; 1(5):379–386. PMID: 19808433.3. Priori SG, Blomström-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, et al. 2015 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the task force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J. 2015; 36(41):2793–2867. PMID: 26320108.4. Antzelevitch C. Genetic, molecular and cellular mechanisms underlying the J wave syndromes. Circ J. 2012; 76(5):1054–1065. PMID: 22498570.
Article5. Burashnikov E, Pfeiffer R, Barajas-Martinez H, Delpón E, Hu D, Desai M, et al. Mutations in the cardiac L-type calcium channel associated with inherited J-wave syndromes and sudden cardiac death. Heart Rhythm. 2010; 7(12):1872–1882. PMID: 20817017.
Article6. Chen Q, Kirsch GE, Zhang D, Brugada R, Brugada J, Brugada P, et al. Genetic basis and molecular mechanism for idiopathic ventricular fibrillation. Nature. 1998; 392(6673):293–296. PMID: 9521325.
Article7. Delpón E, Cordeiro JM, Núñez L, Thomsen PE, Guerchicoff A, Pollevick GD, et al. Functional effects of KCNE3 mutation and its role in the development of Brugada syndrome. Circ Arrhythm Electrophysiol. 2008; 1(3):209–218. PMID: 19122847.8. Giudicessi JR, Ye D, Tester DJ, Crotti L, Mugione A, Nesterenko VV, et al. Transient outward current (I(to)) gain-of-function mutations in the KCND3-encoded Kv4.3 potassium channel and Brugada syndrome. Heart Rhythm. 2011; 8(7):1024–1032. PMID: 21349352.
Article9. London B, Michalec M, Mehdi H, Zhu X, Kerchner L, Sanyal S, et al. Mutation in glycerol-3-phosphate dehydrogenase 1 like gene (GPD1-L) decreases cardiac Na+ current and causes inherited arrhythmias. Circulation. 2007; 116(20):2260–2268. PMID: 17967977.10. Medeiros-Domingo A, Tan BH, Crotti L, Tester DJ, Eckhardt L, Cuoretti A, et al. Gain-of-function mutation S422L in the KCNJ8-encoded cardiac K(ATP) channel Kir6.1 as a pathogenic substrate for J-wave syndromes. Heart Rhythm. 2010; 7(10):1466–1471. PMID: 20558321.
Article11. Schulze-Bahr E, Eckardt L, Breithardt G, Seidl K, Wichter T, Wolpert C, et al. Sodium channel gene (SCN5A) mutations in 44 index patients with Brugada syndrome: different incidences in familial and sporadic disease. Hum Mutat. 2003; 21(6):651–652.
Article12. Watanabe H, Koopmann TT, Le Scouarnec S, Yang T, Ingram CR, Schott JJ, et al. Sodium channel β1 subunit mutations associated with Brugada syndrome and cardiac conduction disease in humans. J Clin Invest. 2008; 118(6):2260–2268. PMID: 18464934.
Article13. Antzelevitch C. J wave syndromes: molecular and cellular mechanisms. J Electrocardiol. 2013; 46(6):510–518. PMID: 24011992.
Article14. Hayashi M, Takatsuki S, Maison-Blanche P, Messali A, Haggui A, Milliez P, et al. Ventricular repolarization restitution properties in patients exhibiting type 1 Brugada electrocardiogram with and without inducible ventricular fibrillation. J Am Coll Cardiol. 2008; 51(12):1162–1168. PMID: 18355653.
Article15. Doi A, Takagi M, Maeda K, Tatsumi H, Shimeno K, Yoshiyama M. Conduction delay in right ventricle as a marker for identifying high-risk patients with Brugada syndrome. J Cardiovasc Electrophysiol. 2010; 21(6):688–696. PMID: 20050961.
Article16. Van Malderen SC, Kerkhove D, Theuns DA, Weytjens C, Droogmans S, Tanaka K, et al. Prolonged right ventricular ejection delay identifies high risk patients and gender differences in Brugada syndrome. Int J Cardiol. 2015; 191:90–96. PMID: 25965611.
Article17. Veerakul G, Nademanee K. Brugada syndrome: two decades of progress. Circ J. 2012; 76(12):2713–2722. PMID: 23149437.18. Wilde AA, Postema PG, Di Diego JM, Viskin S, Morita H, Fish JM, et al. The pathophysiological mechanism underlying Brugada syndrome: depolarization versus repolarization. J Mol Cell Cardiol. 2010; 49(4):543–553. PMID: 20659475.19. Papadatos GA, Wallerstein PM, Head CE, Ratcliff R, Brady PA, Benndorf K, et al. Slowed conduction and ventricular tachycardia after targeted disruption of the cardiac sodium channel gene Scn5a. Proc Natl Acad Sci U S A. 2002; 99(9):6210–6215. PMID: 11972032.
Article20. Nademanee K, Raju H, de Noronha SV, Papadakis M, Robinson L, Rothery S, et al. Fibrosis, connexin-43, and conduction abnormalities in the Brugada syndrome. J Am Coll Cardiol. 2015; 66(18):1976–1986. PMID: 26516000.
Article21. Coronel R, Casini S, Koopmann TT, Wilms-Schopman FJ, Verkerk AO, de Groot JR, et al. Right ventricular fibrosis and conduction delay in a patient with clinical signs of Brugada syndrome: a combined electrophysiological, genetic, histopathologic, and computational study. Circulation. 2005; 112(18):2769–2777. PMID: 16267250.22. Frustaci A, Priori SG, Pieroni M, Chimenti C, Napolitano C, Rivolta I, et al. Cardiac histological substrate in patients with clinical phenotype of Brugada syndrome. Circulation. 2005; 112(24):3680–3687. PMID: 16344400.
Article23. van Veen TA, Stein M, Royer A, Le Quang K, Charpentier F, Colledge WH, et al. Impaired impulse propagation in Scn5a-knockout mice: combined contribution of excitability, connexin expression, and tissue architecture in relation to aging. Circulation. 2005; 112(13):1927–1935. PMID: 16172272.24. Pierpont GL, DeMaster EG, Cohn JN. Regional differences in adrenergic function within the left ventricle. Am J Physiol. 1984; 246(6 Pt 2):H824–H829. PMID: 6742147.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- COVID-19 Vaccination-Induced Ventricular Fibrillation in an Afebrile Patient With Brugada Syndrome
- Brugada Syndrome
- Brugada Syndrome Presenting With Convulsion in the Emergency Department: A Case Report
- Anesthetic Management of a Patient with Brugada Syndrome: A case report
- Anesthetic Experience for Trans-Sphenoidal Surgery of Pituitary Adenoma on a Patient with Brugada Syndrome: A Case Report