J Pathol Transl Med.
2021 Mar;55(2):145-153. 10.4132/jptm.2021.01.26.
Malignant rhabdoid tumor of the kidney in an adult with loss of INI1 expression and mutation in the SMARCB1 gene
- Affiliations
-
- 1Departments of Pathology, Soonchunhyang University Bucheon Hospital, Soonchunhyang University College of Medicine, Bucheon, Korea
- 2Departments of Urology, Soonchunhyang University Bucheon Hospital, Soonchunhyang University College of Medicine, Bucheon, Korea
- KMID: 2513901
- DOI: http://doi.org/10.4132/jptm.2021.01.26
Abstract
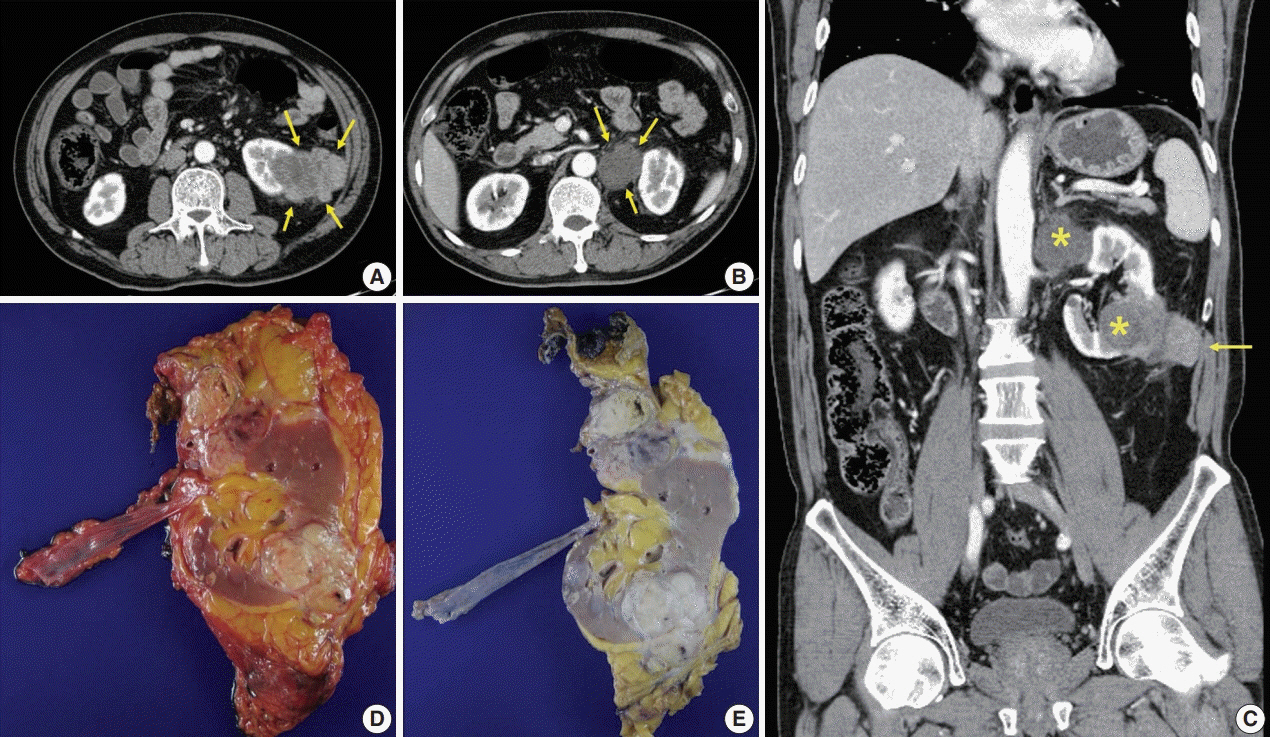
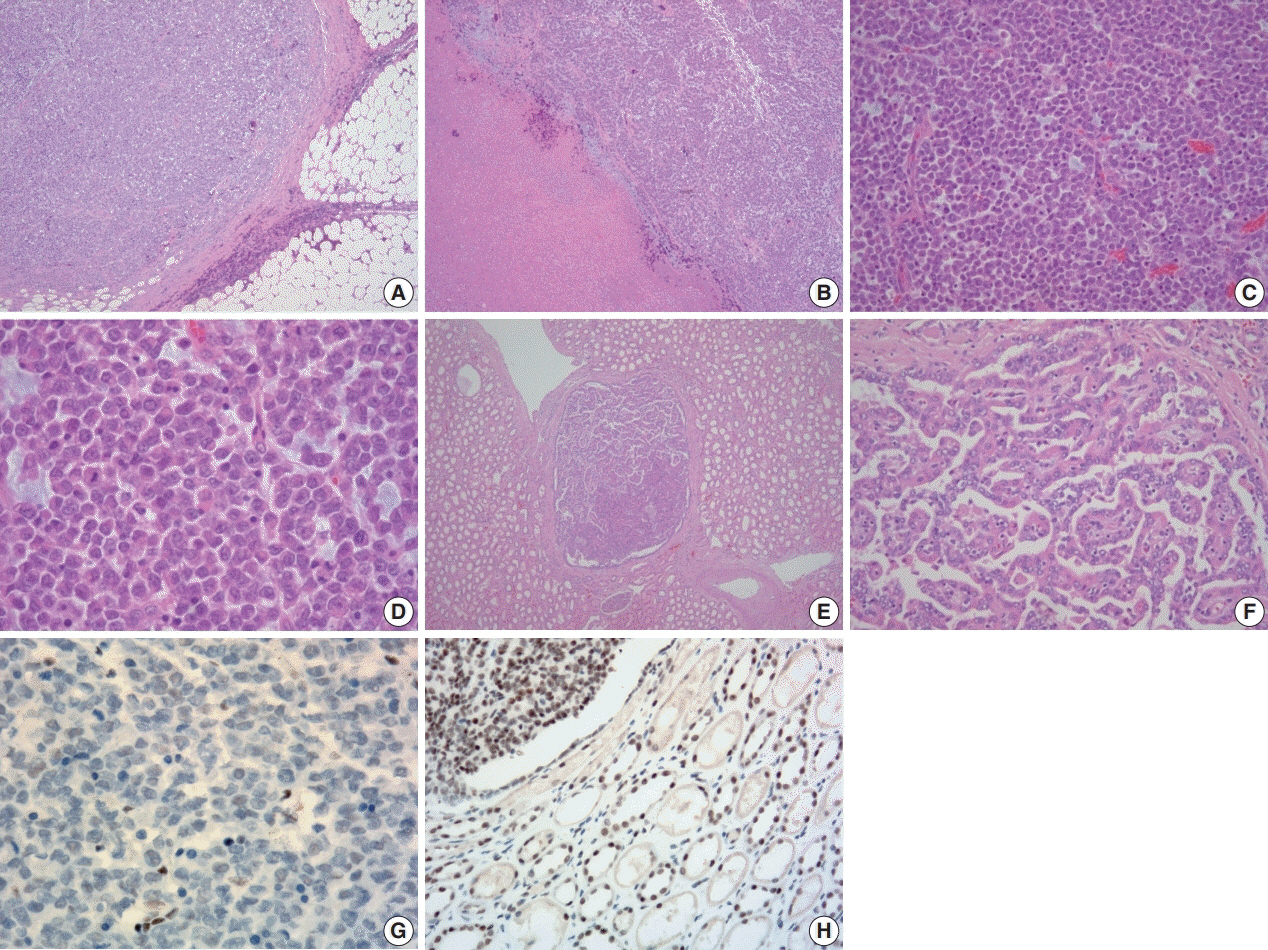
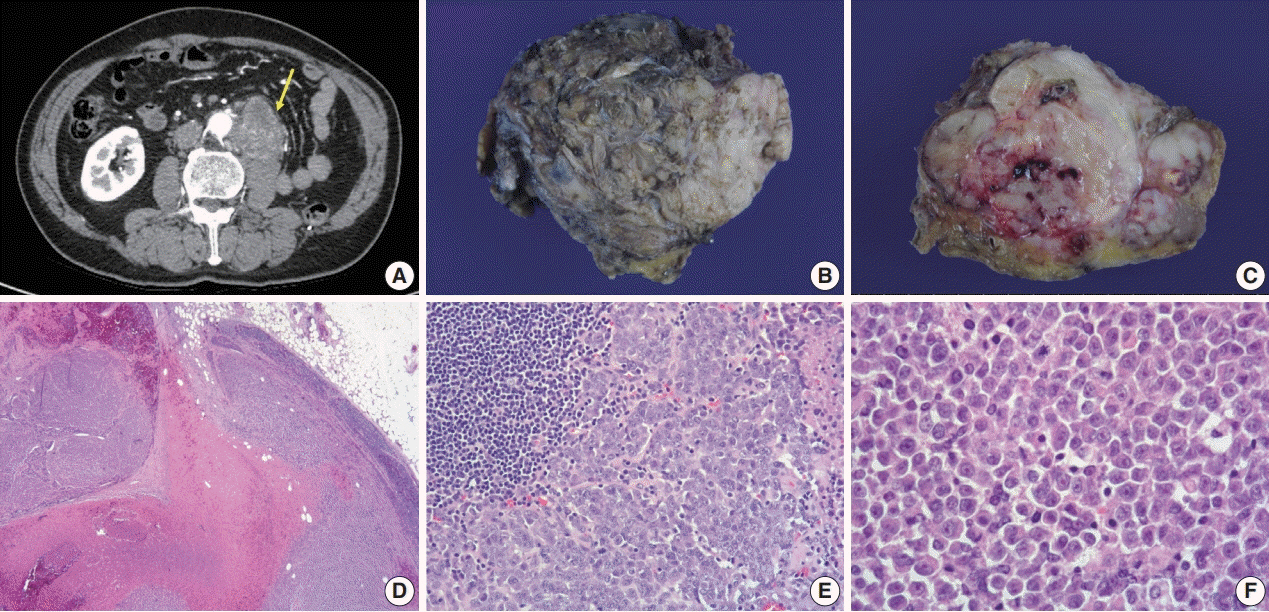
- A 57-year-old man with left flank pain was referred to our institute. Computed tomography scans revealed two enhancing masses in the left kidney. The clinical diagnosis was renal cell carcinoma (RCC). He underwent a radical nephrectomy with an adrenalectomy. Two well-circumscribed solid masses in the hilum and the lower pole (4.5 × 3.5 cm and 7.0 × 4.1 cm) were present. Poorly cohesive uniform round to polygonal epithelioid cells making solid sheets accounted for most of the tumor area. The initial diagnosis was RCC, undifferentiated with rhabdoid features. As the tumor showed loss of INI1 expression and a mutation in the SMARCB1 gene on chromosome 22, the revised diagnosis was a malignant rhabdoid tumor (MRT) of the kidney. To date, only a few cases of renal MRT in adults have been reported. To the best of our knowledge, this is the first report of MRT in the native kidney of an adult demonstrating a SMARCB1 gene mutation, a hallmark of MRT.
Keyword
Figure
Reference
-
References
1. Schmidt D, Harms D, Zieger G. Malignant rhabdoid tumor of the kidney. Histopathology, ultrastructure and comments on differential diagnosis. Virchows Arch A Pathol Anat Histopathol. 1982; 398:101–8.
Article2. Lowe W, Weiss RM, Todd MB, True LD. Malignant rhabdoid tumor of the kidney in an adult. J Urol. 1990; 143:110–1.
Article3. Clausen HV, Horn T, Anagnostaki L, Larsen S. Malignant rhabdoid tumour of the kidney in an adult: a case report with immunohistochemical and ultrastructural investigation. Scand J Urol Nephrol Suppl. 1994; 157:123–8.4. Ebbinghaus SW, Herrera G, Marshall ME. Rhabdoid tumor of the kidney in an adult: review of the literature and report of a case responding to interleukin-2. Cancer Biother. 1995; 10:237–41.
Article5. Peng HQ, Stanek AE, Teichberg S, Shepard B, Kahn E. Malignant rhabdoid tumor of the kidney in an adult: a case report and review of the literature. Arch Pathol Lab Med. 2003; 127:e371–3.
Article6. Zhao G, Na R, Yang Y, Han R. Pure malignant rhabdoid tumor of the left kidney in an adult: a case report and review of the literature. Oncol Lett. 2013; 5:1481–4.
Article7. Podduturi V, Campa-Thompson MM, Zhou XJ, Guileyardo JM. Malignant rhabdoid tumor of the kidney arising in an adult patient. Proc (Bayl Univ Med Cent). 2014; 27:239–41.
Article8. Okumura Y, Adachi Y, Shirahase T, et al. Malignant rhabdoid tumour in an adult kidney: a case report. Mol Clin Oncol. 2019; 11:55–8.
Article9. Ayari Y, Ben Rhouma S, Boussaffa H, et al. Malignant rhabdoid tumor in a solitary kidney arising in an adult patient with chronic obstructive renal calculi. Int J Surg Case Rep. 2019; 58:85–7.
Article10. Sultan I, Qaddoumi I, Rodriguez-Galindo C, Nassan AA, Ghandour K, Al-Hussaini M. Age, stage, and radiotherapy, but not primary tumor site, affects the outcome of patients with malignant rhabdoid tumors. Pediatr Blood Cancer. 2010; 54:35–40.
Article11. Cheng JX, Tretiakova M, Gong C, Mandal S, Krausz T, Taxy JB. Renal medullary carcinoma: rhabdoid features and the absence of INI1 expression as markers of aggressive behavior. Mod Pathol. 2008; 21:647–52.
Article12. Gokden N, Nappi O, Swanson PE, et al. Renal cell carcinoma with rhabdoid features. Am J Surg Pathol. 2000; 24:1329–38.13. Beckwith JB, Palmer NF. Histopathology and prognosis of Wilms tumors: results from the First National Wilms’ Tumor Study. Cancer. 1978; 41:1937–48.14. Versteege I, Sevenet N, Lange J, et al. Truncating mutations of hSNF5/INI1 in aggressive paediatric cancer. Nature. 1998; 394:203–6.
Article15. Roberts CW, Galusha SA, McMenamin ME, Fletcher CD, Orkin SH. Haploinsufficiency of Snf5 (integrase interactor 1) predisposes to malignant rhabdoid tumors in mice. Proc Natl Acad Sci U S A. 2000; 97:13796–800.
Article16. Shannon B, Stan Wisniewski Z, Bentel J, Cohen RJ. Adult rhabdoid renal cell carcinoma. Arch Pathol Lab Med. 2002; 126:1506–10.
Article17. Wick MR, Ritter JH, Dehner LP. Malignant rhabdoid tumors: a clinicopathologic review and conceptual discussion. Semin Diagn Pathol. 1995; 12:233–48.18. Perry A, Fuller CE, Judkins AR, Dehner LP, Biegel JA. INI1 expression is retained in composite rhabdoid tumors, including rhabdoid meningiomas. Mod Pathol. 2005; 18:951–8.
Article19. Przybycin CG, McKenney JK, Reynolds JP, et al. Rhabdoid differentiation is associated with aggressive behavior in renal cell carcinoma: a clinicopathologic analysis of 76 cases with clinical follow-up. Am J Surg Pathol. 2014; 38:1260–5.20. Weeks DA, Beckwith JB, Mierau GW. Rhabdoid tumor: an entity or a phenotype? Arch Pathol Lab Med. 1989; 113:113–4.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Rhabdoid Carcinoma of the Rectum
- Imaging Findings of a Malignant Rhabdoid Tumor in the Stomach: A Case Report
- A Case of Malignant Rhabdoid Tumor in the Anterior Mediastinum
- Epithelioid Malignant Intracerebral Nerve Sheath Tumor: A Case Report and a Comparison with Conventional Type
- Adult-Onset Sellar and Suprasellar Atypical Teratoid Rhabdoid Tumor Treated with a Multimodal Approach: A Case Report