J Pathol Transl Med.
2021 Mar;55(2):83-93. 10.4132/jptm.2021.02.17.
Non-conventional dysplastic subtypes in inflammatory bowel disease: a review of their diagnostic characteristics and potential clinical implications
- Affiliations
-
- 1Department of Pathology, University of California at San Francisco, San Francisco, CA, USA
- KMID: 2513893
- DOI: http://doi.org/10.4132/jptm.2021.02.17
Abstract
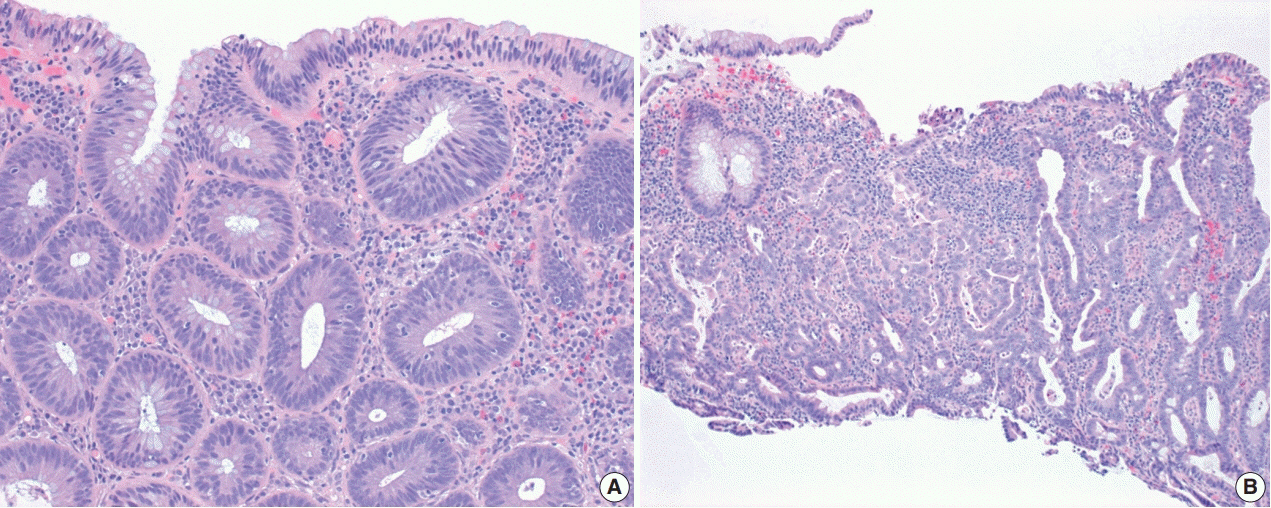
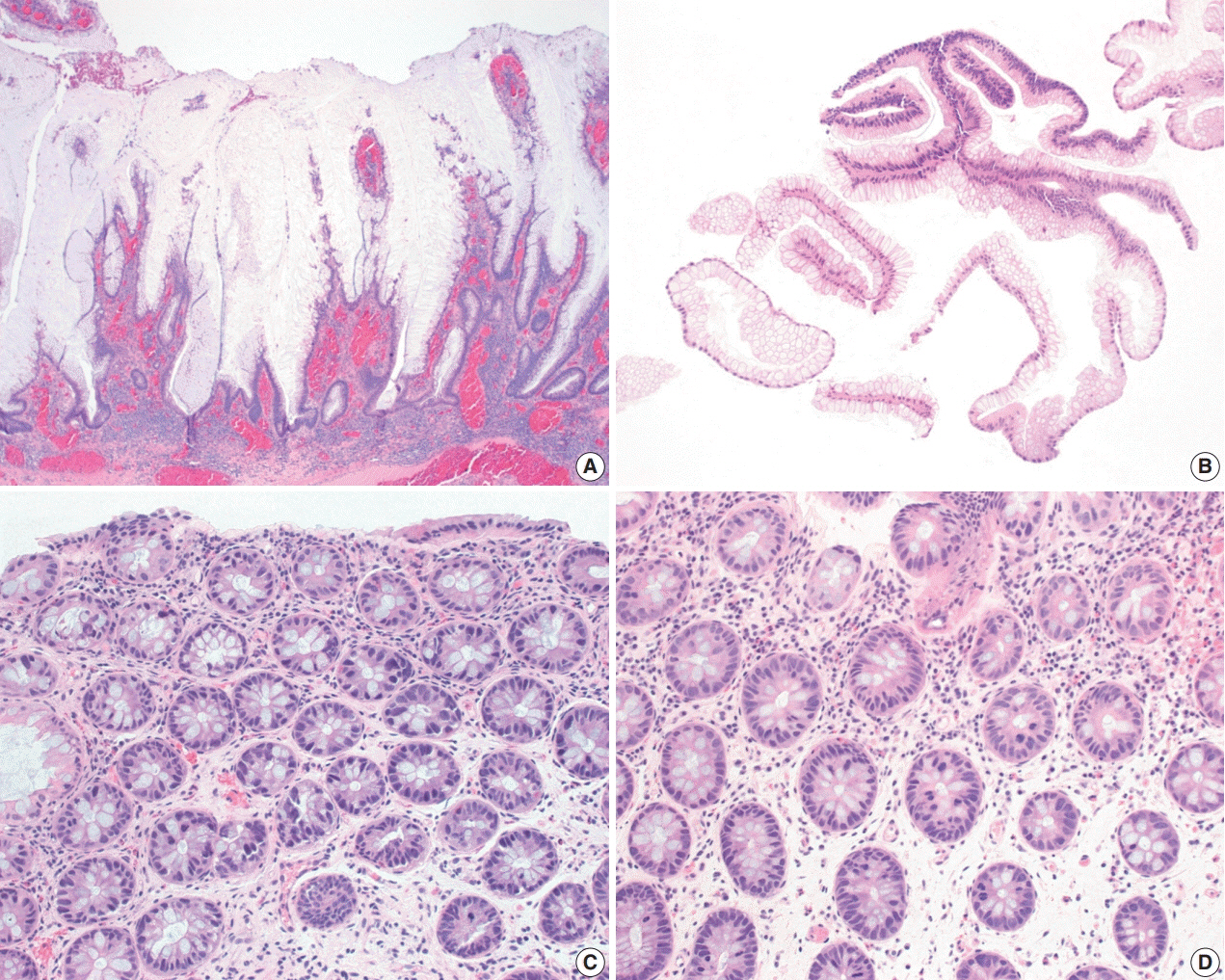
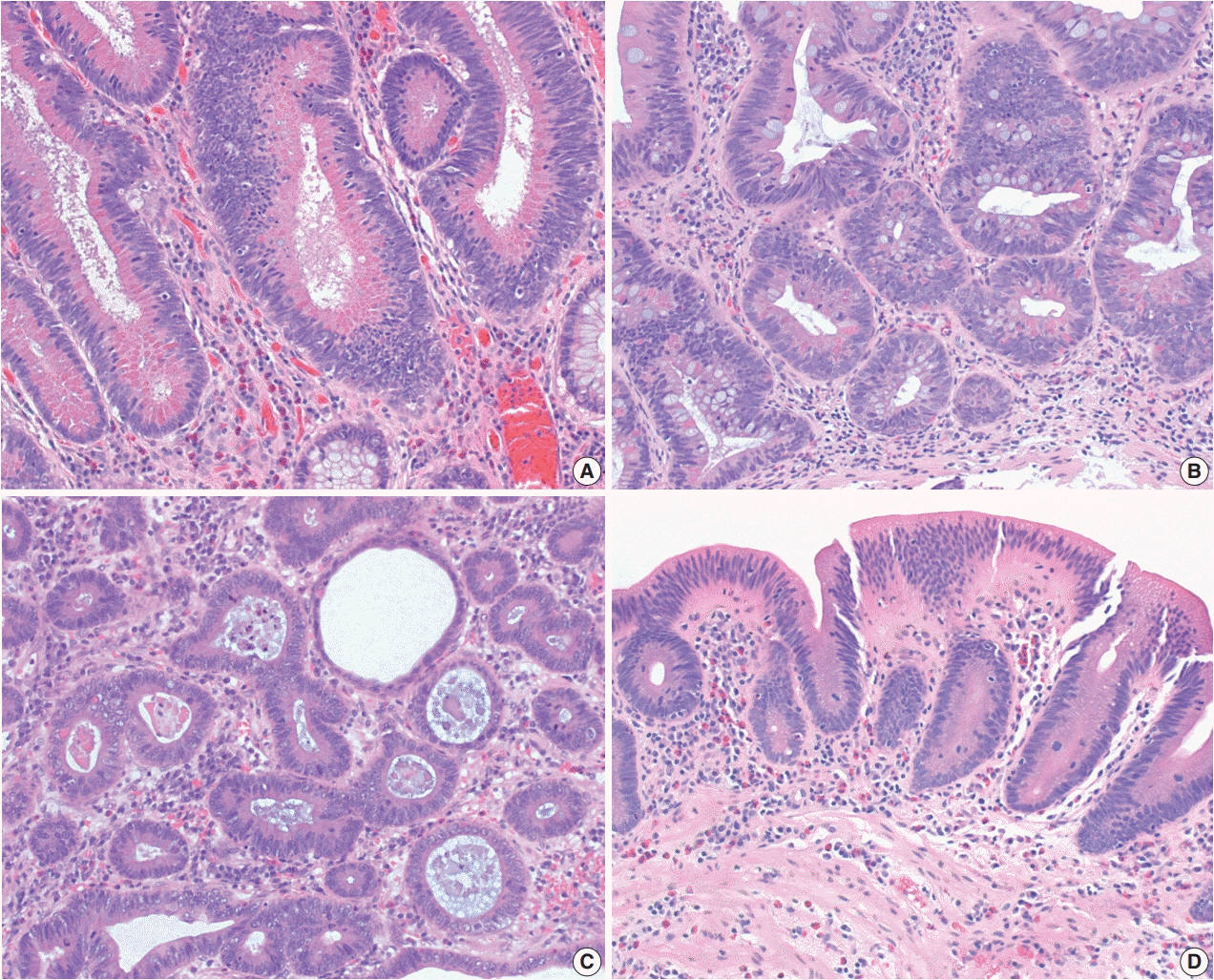
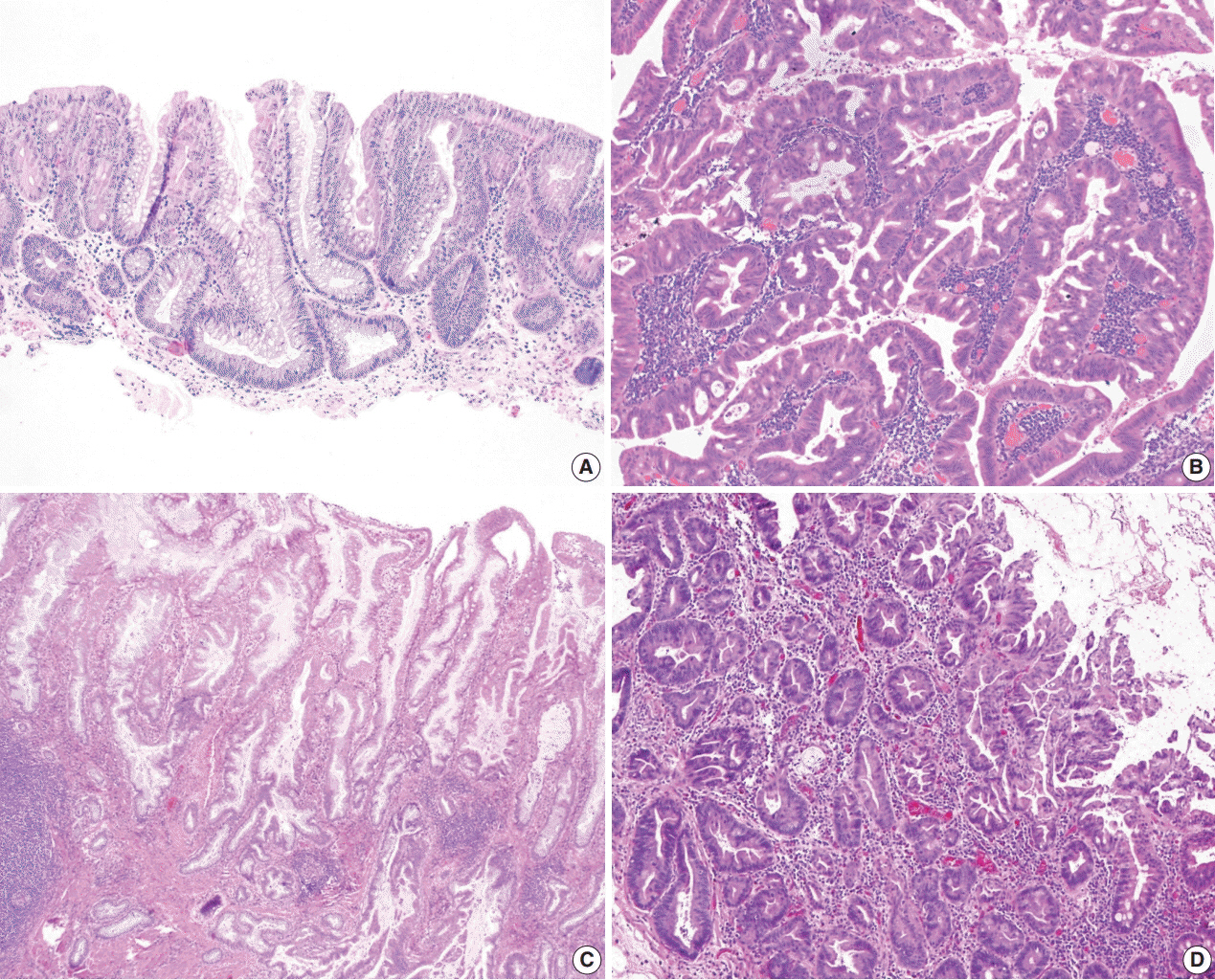
- The early detection and grading of dysplasia is the current standard of care to minimize mortality from colorectal cancer (CRC) in patients with inflammatory bowel disease. With the development of advanced endoscopic resection techniques, colectomy is now reserved for patients with invisible/flat dysplasia (either high-grade [HGD] or multifocal low-grade dysplasia) or endoscopically unresectable lesions. Although most pathologists are familiar with the morphologic criteria of conventional (intestinal type) dysplasia, the most well-recognized form of dysplasia, an increasing number of diagnostic material has led to the recognition of several different morphologic patterns of epithelial dysplasia. The term “non-conventional” dysplasia has been coined to describe these changes, but to date, the recognition and full appreciation of these novel forms of dysplasia by practicing pathologists is uneven. The recognition of these non-conventional subtypes is becoming increasingly important, as some of them appear to have a higher risk of developing HGD or CRC than conventional dysplasia or sporadic adenomas. This review describes the morphologic characteristics of all seven non-conventional subtypes that have been reported to date as well as our current understanding of their clinicopathologic and molecular features that distinguish them from conventional dysplasia or sporadic adenomas.
Figure
Cited by 1 articles
-
And the story goes on: non-conventional dysplasia of the colorectum
Lavisha S. Punjabi, Yi Neng Lai, Anjula Thomas
J Pathol Transl Med. 2022;56(2):109-110. doi: 10.4132/jptm.2021.12.29.
Reference
-
References
1. Ekbom A, Helmick C, Zack M, Adami HO. Ulcerative colitis and colorectal cancer: a population-based study. N Engl J Med. 1990; 323:1228–33.2. Soderlund S, Brandt L, Lapidus A, et al. Decreasing time-trends of colorectal cancer in a large cohort of patients with inflammatory bowel disease. Gastroenterology. 2009; 136:1561–7.
Article3. Bernstein CN, Blanchard JF, Kliewer E, Wajda A. Cancer risk in patients with inflammatory bowel disease: a population-based study. Cancer. 2001; 91:854–62.4. Jess T, Rungoe C, Peyrin-Biroulet L. Risk of colorectal cancer in patients with ulcerative colitis: a meta-analysis of population-based cohort studies. Clin Gastroenterol Hepatol. 2012; 10:639–45.
Article5. Ekbom A, Helmick C, Zack M, Adami HO. Increased risk of large-bowel cancer in Crohn's disease with colonic involvement. Lancet. 1990; 336:357–9.
Article6. Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001; 48:526–35.
Article7. Soetikno RM, Lin OS, Heidenreich PA, Young HS, Blackstone MO. Increased risk of colorectal neoplasia in patients with primary sclerosing cholangitis and ulcerative colitis: a meta-analysis. Gastrointest Endosc. 2002; 56:48–54.
Article8. Beaugerie L, Svrcek M, Seksik P, et al. Risk of colorectal high-grade dysplasia and cancer in a prospective observational cohort of patients with inflammatory bowel disease. Gastroenterology. 2013; 145:166–75.
Article9. Lutgens MW, Oldenburg B, Siersema PD, et al. Colonoscopic surveillance improves survival after colorectal cancer diagnosis in inflammatory bowel disease. Br J Cancer. 2009; 101:1671–5.10. Karlen P, Kornfeld D, Brostrom O, Lofberg R, Persson PG, Ekbom A. Is colonoscopic surveillance reducing colorectal cancer mortality in ulcerative colitis? A population based case control study. Gut. 1998; 42:711–4.
Article11. Eaden J, Abrams K, Ekbom A, Jackson E, Mayberry J. Colorectal cancer prevention in ulcerative colitis: a case-control study. Aliment Pharmacol Ther. 2000; 14:145–53.
Article12. Choi CH, Rutter MD, Askari A, et al. Forty-year analysis of colonoscopic surveillance program for neoplasia in ulcerative colitis: an updated overview. Am J Gastroenterol. 2015; 110:1022–34.
Article13. Laine L, Kaltenbach T, Barkun A, et al. SCENIC international consensus statement on surveillance and management of dysplasia in inflammatory bowel disease. Gastrointest Endosc. 2015; 81:489–501.14. Farraye FA, Odze RD, Eaden J, et al. AGA medical position statement on the diagnosis and management of colorectal neoplasia in inflammatory bowel disease. Gastroenterology. 2010; 138:738–45.
Article15. Leighton JA, Shen B, Baron TH, et al. ASGE guideline: endoscopy in the diagnosis and treatment of inflammatory bowel disease. Gastrointest Endosc. 2006; 63:558–65.
Article16. Odze RD. Adenomas and adenoma-like DALMs in chronic ulcerative colitis: a clinical, pathological, and molecular review. Am J Gastroenterol. 1999; 94:1746–50.
Article17. Rutter MD, Saunders BP, Wilkinson KH, Kamm MA, Williams CB, Forbes A. Most dysplasia in ulcerative colitis is visible at colonoscopy. Gastrointest Endosc. 2004; 60:334–9.
Article18. Rubin DT, Rothe JA, Hetzel JT, Cohen RD, Hanauer SB. Are dysplasia and colorectal cancer endoscopically visible in patients with ulcerative colitis? Gastrointest Endosc. 2007; 65:998–1004.
Article19. Wanders LK, Dekker E, Pullens B, Bassett P, Travis SP, East JE. Cancer risk after resection of polypoid dysplasia in patients with longstanding ulcerative colitis: a meta-analysis. Clin Gastroenterol Hepatol. 2014; 12:756–64.
Article20. Blonski W, Kundu R, Furth EF, Lewis J, Aberra F, Lichtenstein GR. High-grade dysplastic adenoma-like mass lesions are not an indication for colectomy in patients with ulcerative colitis. Scand J Gastroenterol. 2008; 43:817–20.
Article21. Odze RD, Farraye FA, Hecht JL, Hornick JL. Long-term follow-up after polypectomy treatment for adenoma-like dysplastic lesions in ulcerative colitis. Clin Gastroenterol Hepatol. 2004; 2:534–41.
Article22. Kisiel JB, Loftus EV Jr, Harmsen WS, Zinsmeister AR, Sandborn WJ. Outcome of sporadic adenomas and adenoma-like dysplasia in patients with ulcerative colitis undergoing polypectomy. Inflamm Bowel Dis. 2012; 18:226–35.
Article23. Connell WR, Lennard-Jones JE, Williams CB, Talbot IC, Price AB, Wilkinson KH. Factors affecting the outcome of endoscopic surveillance for cancer in ulcerative colitis. Gastroenterology. 1994; 107:934–44.
Article24. Friedman S, Rubin PH, Bodian C, Goldstein E, Harpaz N, Present DH. Screening and surveillance colonoscopy in chronic Crohn's colitis. Gastroenterology. 2001; 120:820–6.
Article25. Hata K, Watanabe T, Kazama S, et al. Earlier surveillance colonoscopy programme improves survival in patients with ulcerative colitis associated colorectal cancer: results of a 23-year surveillance programme in the Japanese population. Br J Cancer. 2003; 89:1232–6.
Article26. Bernstein CN, Shanahan F, Weinstein WM. Are we telling patients the truth about surveillance colonoscopy in ulcerative colitis? Lancet. 1994; 343:71–4.27. Thomas T, Abrams KA, Robinson RJ, Mayberry JF. Meta-analysis: cancer risk of low-grade dysplasia in chronic ulcerative colitis. Aliment Pharmacol Ther. 2007; 25:657–68.
Article28. Jess T, Loftus EV Jr, Velayos FS, et al. Incidence and prognosis of colorectal dysplasia in inflammatory bowel disease: a population-based study from Olmsted County, Minnesota. Inflamm Bowel Dis. 2006; 12:669–76.
Article29. Ullman T, Croog V, Harpaz N, Sachar D, Itzkowitz S. Progression of flat low-grade dysplasia to advanced neoplasia in patients with ulcerative colitis. Gastroenterology. 2003; 125:1311–9.
Article30. Ullman TA, Loftus EV Jr, Kakar S, Burgart LJ, Sandborn WJ, Tremaine WJ. The fate of low grade dysplasia in ulcerative colitis. Am J Gastroenterol. 2002; 97:922–7.
Article31. van Schaik FD, ten Kate FJ, Offerhaus GJ, et al. Misclassification of dysplasia in patients with inflammatory bowel disease: consequences for progression rates to advanced neoplasia. Inflamm Bowel Dis. 2011; 17:1108–16.32. Befrits R, Ljung T, Jaramillo E, Rubio C. Low-grade dysplasia in extensive, long-standing inflammatory bowel disease: a follow-up study. Dis Colon Rectum. 2002; 45:615–20.33. Venkatesh PG, Jegadeesan R, Gutierrez NG, Sanaka MR, Navaneethan U. Natural history of low grade dysplasia in patients with primary sclerosing cholangitis and ulcerative colitis. J Crohns Colitis. 2013; 7:968–73.
Article34. Pekow JR, Hetzel JT, Rothe JA, et al. Outcome after surveillance of low-grade and indefinite dysplasia in patients with ulcerative colitis. Inflamm Bowel Dis. 2010; 16:1352–6.
Article35. Zisman TL, Bronner MP, Rulyak S, et al. Prospective study of the progression of low-grade dysplasia in ulcerative colitis using current cancer surveillance guidelines. Inflamm Bowel Dis. 2012; 18:2240–6.
Article36. Navaneethan U, Jegadeesan R, Gutierrez NG, et al. Progression of low-grade dysplasia to advanced neoplasia based on the location and morphology of dysplasia in ulcerative colitis patients with extensive colitis under colonoscopic surveillance. J Crohns Colitis. 2013; 7:e684–91.
Article37. Tsai JH, Rabinovitch PS, Huang D, et al. Association of aneuploidy and flat dysplasia with development of high-grade dysplasia or colorectal cancer in patients with inflammatory bowel disease. Gastroenterology. 2017; 153:1492–5.
Article38. Lee H, Rabinovitch PS, Mattis AN, Lauwers GY, Choi WT. Nonconventional dysplasia in inflammatory bowel disease is more frequently associated with advanced neoplasia and aneuploidy than conventional dysplasia. Histopathology 2020 Nov 5 [Epub]. https://doi.org/10.1111/his.14298.
Article39. Wanders LK, Cordes M, Voorham Q, et al. IBD-associated dysplastic lesions show more chromosomal instability than sporadic adenomas. Inflamm Bowel Dis. 2020; 26:167–80.
Article40. Riddell RH, Goldman H, Ransohoff DF, et al. Dysplasia in inflammatory bowel disease: standardized classification with provisional clinical applications. Hum Pathol. 1983; 14:931–68.
Article41. Choi WT, Yozu M, Miller GC, et al. Nonconventional dysplasia in patients with inflammatory bowel disease and colorectal carcinoma: a multicenter clinicopathologic study. Mod Pathol. 2020; 33:933–43.
Article42. Wen KW, Umetsu SE, Goldblum JR, et al. DNA flow cytometric and interobserver study of crypt cell atypia in inflammatory bowel disease. Histopathology. 2019; 75:578–88.
Article43. Pereira D, Kovari B, Brown I, et al. Non-conventional dysplasias of the tubular gut: a review and illustration of their histomorphological spectrum. Histopathology 2020 Oct 30 [Epub]. https://doi.org/10.1111/his.14294.
Article44. Watanabe T, Ajioka Y, Mitsuyama K, et al. Comparison of targeted vs random biopsies for surveillance of ulcerative colitis-associated colorectal cancer. Gastroenterology. 2016; 151:1122–30.45. Andersen SN, Lovig T, Clausen OP, Bakka A, Fausa O, Rognum TO. Villous, hypermucinous mucosa in long standing ulcerative colitis shows high frequency of K-ras mutations. Gut. 1999; 45:686–92.
Article46. Maltzman T, Knoll K, Martinez ME, et al. Ki-ras proto-oncogene mutations in sporadic colorectal adenomas: relationship to histologic and clinical characteristics. Gastroenterology. 2001; 121:302–9.
Article47. Gui X, Kobel M, Ferraz JG, et al. Histological and molecular diversity and heterogeneity of precancerous lesions associated with inflammatory bowel diseases. J Clin Pathol. 2020; 73:391–402.
Article48. Vogelstein B, Fearon ER, Hamilton SR, et al. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988; 319:525–32.
Article49. Ried T, Knutzen R, Steinbeck R, et al. Comparative genomic hybridization reveals a specific pattern of chromosomal gains and losses during the genesis of colorectal tumors. Genes Chromosomes Cancer. 1996; 15:234–45.
Article50. Meijer GA, Hermsen MA, Baak JP, et al. Progression from colorectal adenoma to carcinoma is associated with non-random chromosomal gains as detected by comparative genomic hybridisation. J Clin Pathol. 1998; 51:901–9.51. Lomo LC, Blount PL, Sanchez CA, et al. Crypt dysplasia with surface maturation: a clinical, pathologic, and molecular study of a Barrett’s esophagus cohort. Am J Surg Pathol. 2006; 30:423–35.52. Joo M, Shahsafaei A, Odze RD. Paneth cell differentiation in colonic epithelial neoplasms: evidence for the role of the Apc/beta-catenin/ Tcf pathway. Hum Pathol. 2009; 40:872–80.53. Mahon M, Xu J, Yi X, Liu X, Gao N, Zhang L. Paneth cell in adenomas of the distal colorectum is inversely associated with synchronous advanced adenoma and carcinoma. Sci Rep. 2016; 6:26129.
Article54. Bansal M, Fenoglio CM, Robboy SJ, King DW. Are metaplasias in colorectal adenomas truly metaplasias? Am J Pathol. 1984; 115:253–65.55. Pai RK, Rybicki LA, Goldblum JR, Shen B, Xiao SY, Liu X. Paneth cells in colonic adenomas: association with male sex and adenoma burden. Am J Surg Pathol. 2013; 37:98–103.56. Ko HM, Harpaz N, McBride RB, et al. Serrated colorectal polyps in inflammatory bowel disease. Mod Pathol. 2015; 28:1584–93.
Article57. Yang C, Tarabishy Y, Dassopoulos T, Nalbantoglu I. Clinical, histologic, and immunophenotypic features of serrated polyps in patients with inflammatory bowel disease. Gastroenterology Res. 2018; 11:355–60.
Article58. Choi WT, Wen KW, Rabinovitch PS, Huang D, Mattis AN, Gill RM. DNA content analysis of colorectal serrated lesions detects an aneuploid subset of inflammatory bowel disease-associated serrated epithelial change and traditional serrated adenomas. Histopathology. 2018; 73:464–72.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Inflammatory Bowel Disease in Pediatric Age
- Abdominal Pain over 6 Months
- Inflammatory Bowel Disease in Korea: Epidemiological, Genomic, Clinical, and Therapeutic Characteristics
- Natural Product-Derived Drugs for the Treatment of Inflammatory Bowel Diseases
- Artificial intelligence in inflammatory bowel disease: implications for clinical practice and future directions