J Korean Neurosurg Soc.
2021 Mar;64(2):297-308. 10.3340/jkns.2019.0219.
Systematic Review and Meta-Analysis of Antibiotic-Impregnated Shunt Catheters on Anti-Infective Effect of Hydrocephalus Shunt
- Affiliations
-
- 1National United Engineering Laboratory for Biomedical Material Modification, Branden Industrial Park, Dezhou, China
- 2Department of Interventional Neuroradiology, Beijing Tiantan Hospital, Capital Medical University, Beijing, China
- 3Beijing Neurosurgical Institute, Capital Medical University, Beijing, China
- 4China National Clinical Research Center for Neurological Diseases, Beijing, China
- 5Department of Vascular & Intervention, Tenth People’s Hospital of Tongji University, Shanghai, China
- 6Department of Health Science and Technology, Faculty of Medicine, Aalborg University, Alborg, Denmark
- KMID: 2513861
- DOI: http://doi.org/10.3340/jkns.2019.0219
Abstract
Objective
: Shunt infection is a common complication while treating hydrocephalus. The antibiotic-impregnated shunt catheter (AISC) was designed to reduce shunt infection rate. A meta-analysis was conducted to study the effectiveness of AISCs in reduction of shunt infection in terms of age, follow-up time and high-risk patient population.
Methods
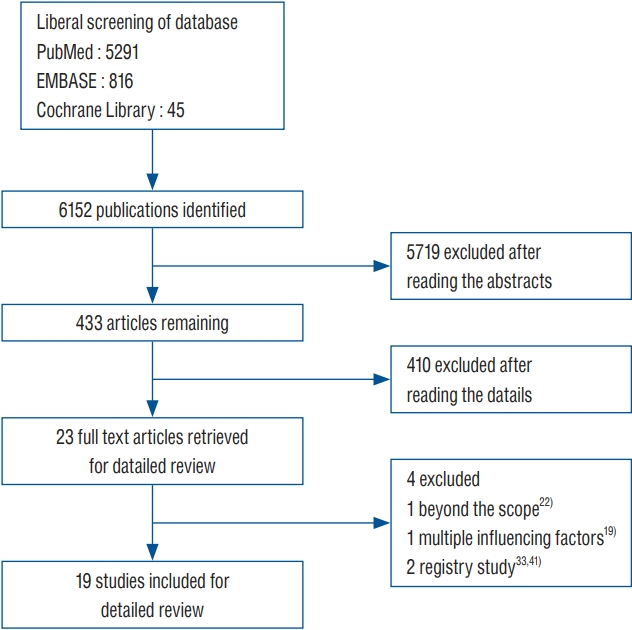
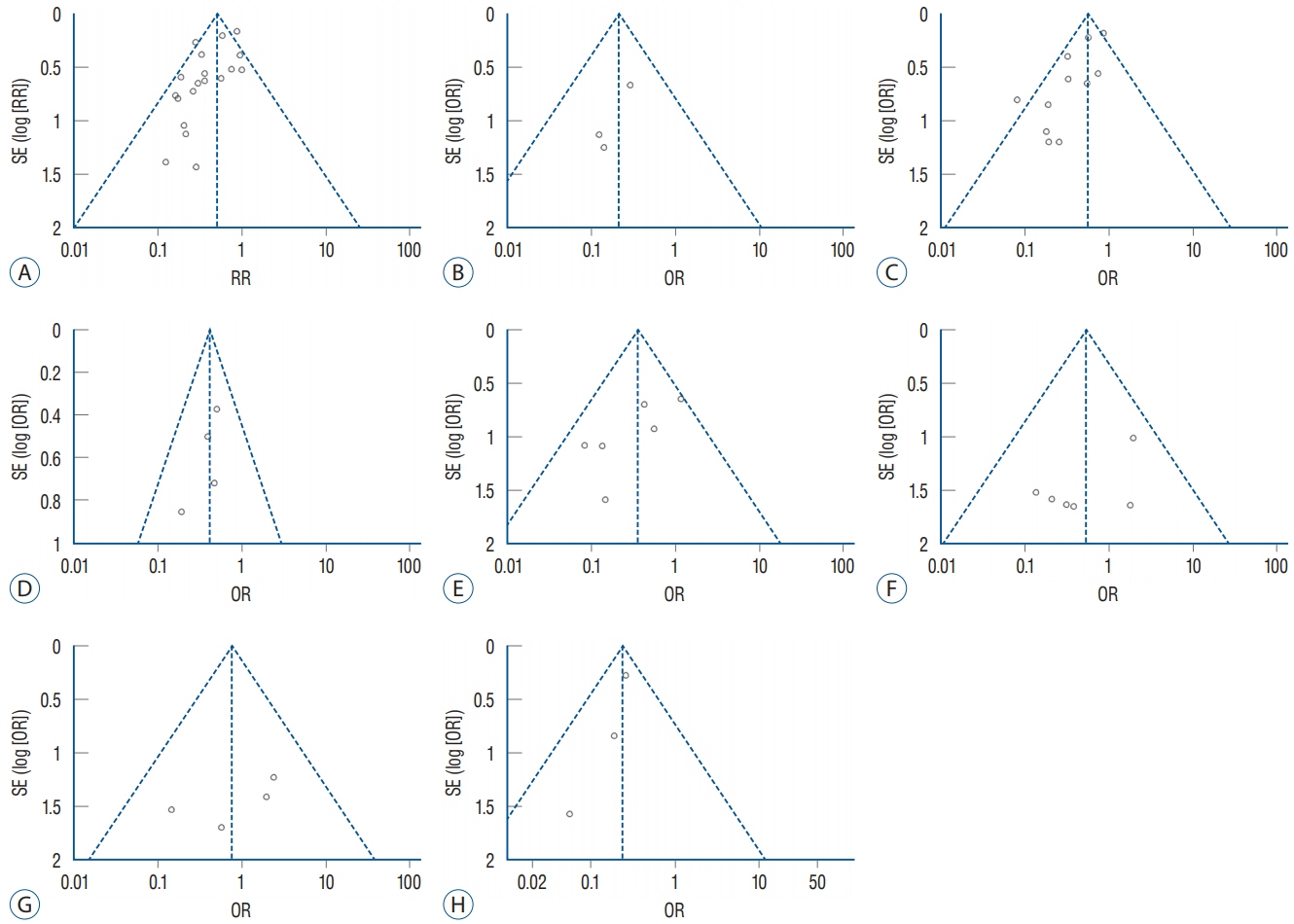
: This study reviewed literature from three databases including PubMed, EMBASE, and Cochrane Library (from 2000 to March 2019). Clinical studies from controlled trials for shunt operation were included in this analysis. A subgroup analysis was performed based on the patient’s age, follow-up time and high-risk population. The fixed effect in RevMan 5.3 software (Cochrane Collaboration) was used for this meta-analysis.
Results
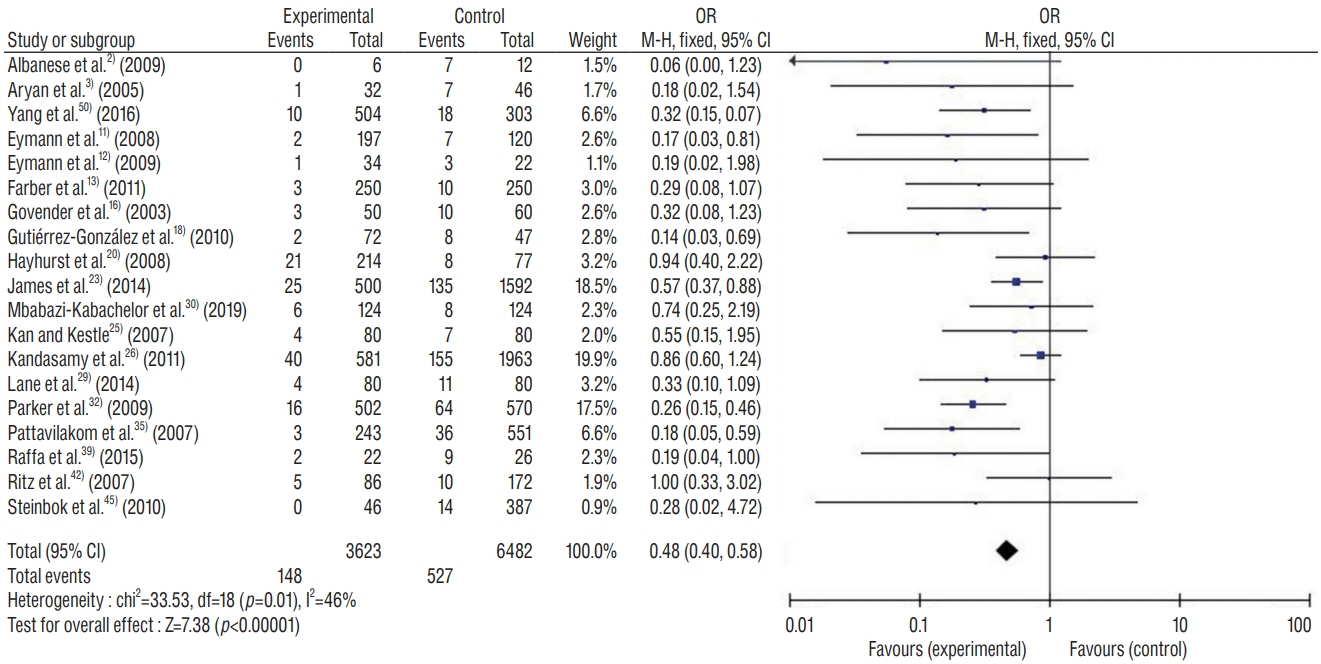
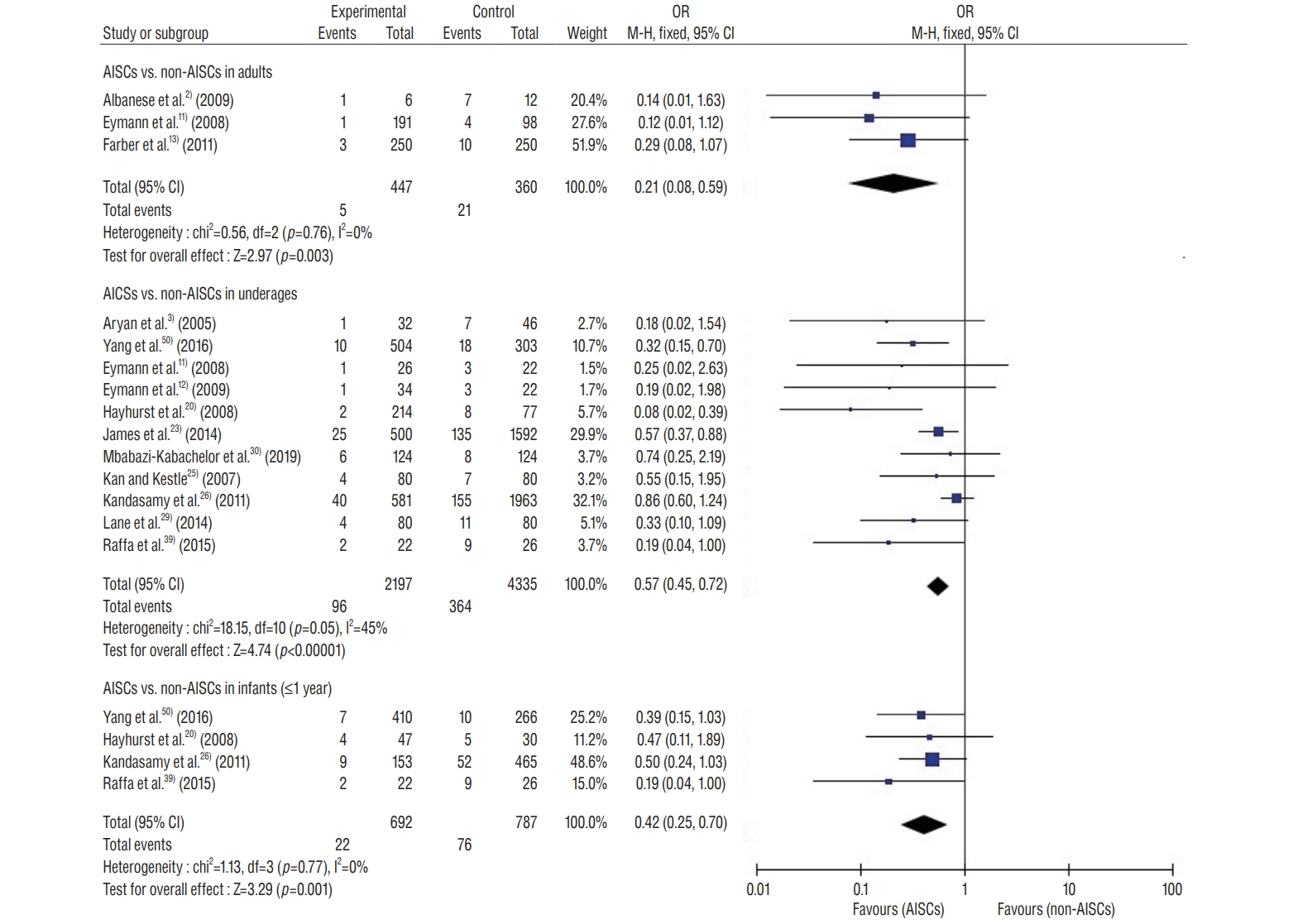
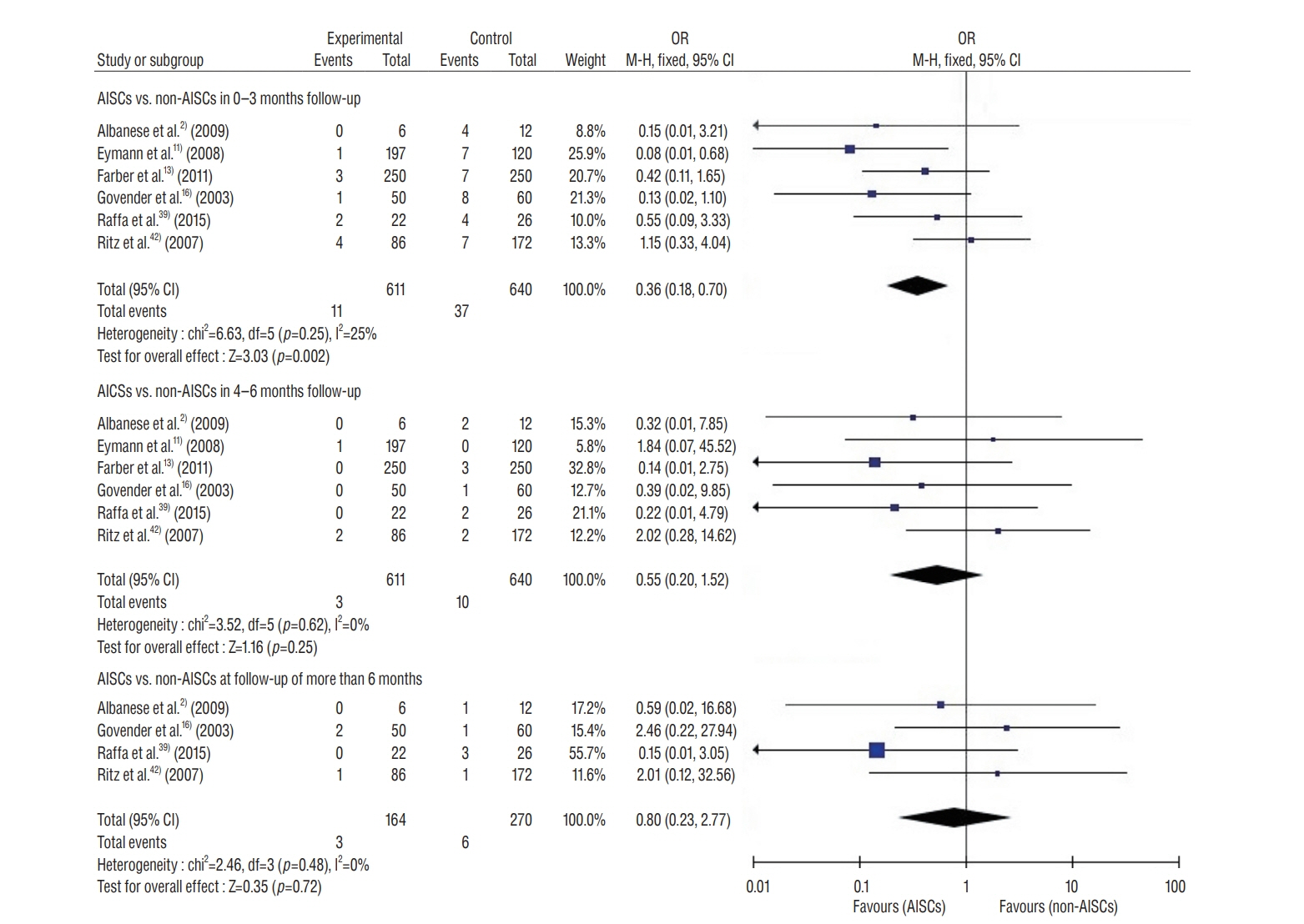
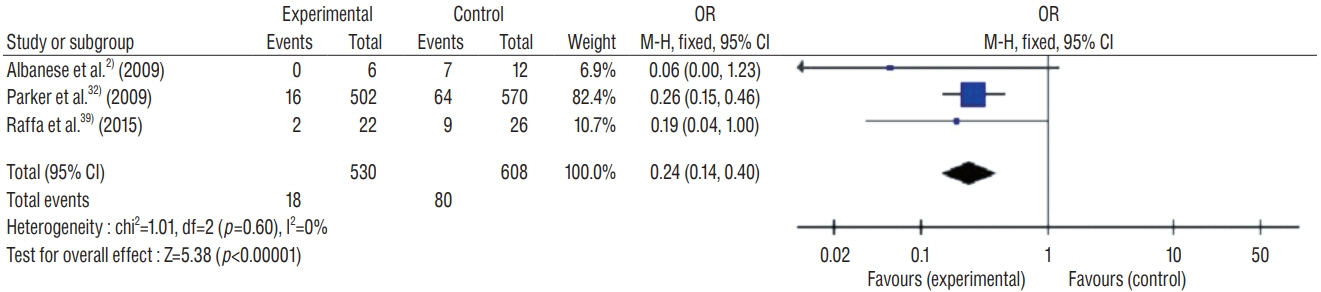
: This study included 19 controlled clinical trials including 10105 operations. The analysis demonstrated that AISC could reduce the infection rate in shunt surgery compared to standard shunt catheter (non-AISC) from 8.13% to 4.09% (odds ratio [OR], 0.48; 95% confidence interval [CI], 0.40–0.58; p=0.01; I2=46%). Subgroup analysis of different age groups showed that AISC had significant antimicrobial effects in all three groups (adult, infant, and adolescent). Follow-up time analysis showed that AISC was effective in preventing early shunt infections (within 6 months after implant). AISC is more effective in high-risk population (OR, 0.24; 95% CI, 0.14–0.40; p=0.60; I2=0%) than in general patient population.
Conclusion
: The results of meta-analysis indicated that AISC is an effective method for reducing shunt infection. We recommend that AISC should be considered for use in infants and high-risk groups. For adult patients, the choice for AISC could be determined based on the treatment cost.
Figure
Reference
-
References
1. Adams DJ, Rajnik M. Microbiology and treatment of cerebrospinal fluid shunt infections in children. Curr Infect Dis Rep. 16:427. 2014.
Article2. Albanese A, De Bonis P, Sabatino G, Capone G, Marchese E, Vignati A, et al. Antibiotic-impregnated ventriculo-peritoneal shunts in patients at high risk of infection. Acta Neurochir (Wien). 151:1259–1263. 2009.
Article3. Aryan HE, Meltzer HS, Park MS, Bennett RL, Jandial R, Levy ML. Initial experience with antibiotic-impregnated silicone catheters for shunting of cerebrospinal fluid in children. Childs Nerv Syst. 21:56–61. 2005.
Article4. Bayston R. Hydrocephalus shunt infections. J Antimicrob Chemother. 34 Suppl A:75–84. 1994.
Article5. Bayston R, Grove N, Siegel J, Lawellin D, Barsham S. Prevention of hydrocephalus shunt catheter colonisation in vitro by impregnation with antimicrobials. J Neurol Neurosurg Psychiatry. 52:605–609. 1989.
Article6. Bayston R, Lari J. A study of the sources of infection in colonised shunts. Dev Med Child Neurol. 16(6 Suppl 32):16–22. 1974.
Article7. Braga MH, Carvalho GT, Brandão RA, Lima FB, Costa BS. Early shunt complications in 46 children with hydrocephalus. Arq Neuropsiquiatr. 67(2A):273–277. 2009.
Article8. Choux M, Genitori L, Lang D, Lena G. Shunt implantation: reducing the incidence of shunt infection. J Neurosurg. 77:875–880. 1992.
Article9. Choux M, Lena G, Genitori L, Empime E, Riss JM. Shunt Implantation-Toward Zero Infection : Childs Nervous System. New York: Springer;1988. p. 181.10. Enger PØ, Svendsen F, Wester K. CSF shunt infections in children: experiences from a population-based study. Acta Neurochir (Wien). 145:243–248. discussion 248. 2003.
Article11. Eymann R, Chehab S, Strowitzki M, Steudel WI, Kiefer M. Clinical and economic consequences of antibiotic-impregnated cerebrospinal fluid shunt catheters. J Neurosurg Pediatr. 1:444–450. 2008.
Article12. Eymann R, Steudel WI, Kiefer M. Infection rate with application of an antibiotic-impregnated catheter for shunt implantation in children - a retrospective analysis. Klin Padiatr. 221:69–73. 2009.
Article13. Farber SH, Parker SL, Adogwa O, McGirt MJ, Rigamonti D. Effect of antibiotic-impregnated shunts on infection rate in adult hydrocephalus: a single institution’s experience. Neurosurgery. 69:625–629. discussion 629. 2011.
Article14. Farber SH, Parker SL, Adogwa O, Rigamonti D, McGirt MJ. Cost analysis of antibiotic-impregnated catheters in the treatment of hydrocephalus in adult patients. World Neurosurg. 74:528–531. 2010.
Article15. George R, Leibrock L, Epstein M. Long-term analysis of cerebrospinal fluid shunt infections. A 25-year experience. J Neurosurg. 51:804–811. 1979.16. Govender ST, Nathoo N, van Dellen JR. Evaluation of an antibiotic-impregnated shunt system for the treatment of hydrocephalus. J Neurosurg. 99:831–839. 2003.
Article17. Gutiérrez-González R, Boto GR. Do antibiotic-impregnated catheters prevent infection in CSF diversion procedures? Review of the literature. J Infect. 61:9–20. 2010.
Article18. Gutiérrez-González R, Boto GR, Fernández-Pérez C, del Prado N. Protective effect of rifampicin and clindamycin-impregnated devices against Staphylococcus spp. infection after cerebrospinal fluid diversion procedures. BMC Neurol. 10:93. 2010.19. Harrop JS, Sharan AD, Ratliff J, Prasad S, Jabbour P, Evans JJ, et al. Impact of a standardized protocol and antibiotic-impregnated catheters on ventriculostomy infection rates in cerebrovascular patients. Neurosurgery. 67:187–191. discussion 191. 2010.
Article20. Hayhurst C, Cooke R, Williams D, Kandasamy J, O’Brien DF, Mallucci CL. The impact of antibiotic-impregnated catheters on shunt infection in children and neonates. Childs Nerv Syst. 24:557–562. 2008.
Article21. Higgins JP, Wells GA. Cochrane handbook for systematic reviews of interventions. Hoboken: John Wiley & Sons;2011.22. Jaeger W, Lee S, Vineet D, Keil A, Agarwal N, Rao S. Ventriculoperitoneal shunts in neonates: a retrospective study of outcomes with antibiotic-impregnated catheters and a modified peri-operative antibiotic protocol. Br J Neurosurg. 31:672–676. 2017.
Article23. James G, Hartley JC, Morgan RD, Ternier J. Effect of introduction of antibiotic-impregnated shunt catheters on cerebrospinal fluid shunt infection in children: a large single-center retrospective study. J Neurosurg Pediatr. 13:101–106. 2014.
Article24. James HE, Bradley JS. Aggressive management of shunt infection: combined intravenous and intraventricular antibiotic therapy for twelve or less days. Pediatr Neurosurg. 44:104–111. 2008.
Article25. Kan P, Kestle J. Lack of efficacy of antibiotic-impregnated shunt systems in preventing shunt infections in children. Childs Nerv Syst. 23:773–777. 2007.
Article26. Kandasamy J, Dwan K, Hartley JC, Jenkinson MD, Hayhurst C, Gatscher S, et al. Antibiotic-impregnated ventriculoperitoneal shunts--a multicentre British paediatric neurosurgery group (BPNG) study using historical controls. Childs Nerv Syst. 27:575–581. 2011.
Article27. Kestle J, Drake J, Milner R, Sainte-Rose C, Cinalli G, Boop F, et al. Longterm follow-up data from the Shunt Design Trial. Pediatr Neurosurg. 33:230–236. 2000.
Article28. Konstantelias AA, Vardakas KZ, Polyzos KA, Tansarli GS, Falagas ME. Antimicrobial-impregnated and -coated shunt catheters for prevention of infections in patients with hydrocephalus: a systematic review and meta-analysis. J Neurosurg. 122:1096–1112. 2015.
Article29. Lane JD, Mugamba J, Ssenyonga P, Warf BC. Effectiveness of the Bactiseal Universal Shunt for reducing shunt infection in a sub-Saharan African context: a retrospective cohort study in 160 Ugandan children. J Neurosurg Pediatr. 13:140–144. 2014.
Article30. Mbabazi-Kabachelor E, Shah M, Vaughan KA, Mugamba J, Ssenyonga P, Onen J, et al. Infection risk for Bactiseal Universal Shunts versus Chhabra shunts in Ugandan infants: a randomized controlled trial. J Neurosurg Pediatr. 23:397–406. 2019.
Article31. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 151:264–269. 2009.
Article32. Parker SL, Attenello FJ, Sciubba DM, Garces-Ambrossi GL, Ahn E, Weingart J, et al. Comparison of shunt infection incidence in high-risk subgroups receiving antibiotic-impregnated versus standard shunts. Childs Nerv Syst. 25:77–83. discussion 85. 2009.
Article33. Parker SL, McGirt MJ, Murphy JA, Megerian JT, Stout M, Engelhart L. Comparative effectiveness of antibiotic-impregnated shunt catheters in the treatment of adult and pediatric hydrocephalus: analysis of 12,589 consecutive cases from 287 US hospital systems. J Neurosurg. 122:443–448. 2015.
Article34. Pattavilakom A, Kotasnas D, Korman TM, Xenos C, Danks A. Duration of in vivo antimicrobial activity of antibiotic-impregnated cerebrospinal fluid catheters. Neurosurgery. 58:930–935. discussion 930-935. 2006.
Article35. Pattavilakom A, Xenos C, Bradfield O, Danks RA. Reduction in shunt infection using antibiotic impregnated CSF shunt catheters: an Australian prospective study. J Clin Neurosci. 14:526–531. 2007.
Article36. Piatt JH Jr. Cerebrospinal fluid shunt failure: late is different from early. Pediatr Neurosurg. 23:133–139. 1995.
Article37. Pople IK, Bayston R, Hayward RD. Infection of cerebrospinal fluid shunts in infants: a study of etiological factors. J Neurosurg. 77:29–36. 1992.
Article38. Prusseit J, Simon M, von der Brelie C, Heep A, Molitor E, Volz S, et al. Epidemiology, prevention and management of ventriculoperitoneal shunt infections in children. Pediatr Neurosurg. 45:325–336. 2009.
Article39. Raffa G, Marseglia L, Gitto E, Germanò A. Antibiotic-impregnated catheters reduce ventriculoperitoneal shunt infection rate in high-risk newborns and infants. Childs Nerv Syst. 31:1129–1138. 2015.
Article40. Rehman AU, Rehman TU, Bashir HH, Gupta V. A simple method to reduce infection of ventriculoperitoneal shunts. J Neurosurg-Pediatr. 5:569–572. 2010.
Article41. Richards HK, Seeley HM, Pickard JD. Efficacy of antibiotic-impregnated shunt catheters in reducing shunt infection: data from the United Kingdom Shunt Registry. J Neurosurg Pediatr. 4:389–393. 2009.
Article42. Ritz R, Roser F, Morgalla M, Dietz K, Tatagiba M, Will BE. Do antibioticimpregnated shunts in hydrocephalus therapy reduce the risk of infection? An observational study in 258 patients. BMC Infect Dis. 7:38. 2007.
Article43. Sciubba DM, McGirt MJ, Woodworth GF, Carson B, Jallo GI. Prolonged exposure to antibiotic-impregnated shunt catheters does not increase incidence of late shunt infections. Childs Nerv Syst. 23:867–871. 2007.
Article44. Sciubba DM, Stuart RM, McGirt MJ, Woodworth GF, Samdani A, Carson B, et al. Effect of antibiotic-impregnated shunt catheters in decreasing the incidence of shunt infection in the treatment of hydrocephalus. J Neurosurg. 103(2 Suppl):131–136. 2005.
Article45. Steinbok P, Milner R, Agrawal D, Farace E, Leung GK, Ng I, et al. A multicenter multinational registry for assessing ventriculoperitoneal shunt infections for hydrocephalus. Neurosurgery. 67:1303–1310. 2010.
Article46. Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 355:i4919. 2016.
Article47. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. 283:2008–2012. 2000.
Article48. Thomas R, Lee S, Patole S, Rao S. Antibiotic-impregnated catheters for the prevention of CSF shunt infections: a systematic review and metaanalysis. Br J Neurosurg. 26:175–184. 2012.
Article49. Vinchon M, Lemaitre MP, Vallée L, Dhellemmes P. Late shunt infection: incidence, pathogenesis, and therapeutic implications. Neuropediatrics. 33:169–173. 2002.
Article50. Yang B, Song Y, Gao P, Bao N. Prevention of infection by antibioticimpregnated shunts after pediatric hydrocephalus treatment: a single center, retrospective study in China. Clin Neurol Neurosurg. 151:92–95. 2016.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Asymptomatic Fracture of a Distal Shunt Catheter after Ventriculoperitoneal Shunt for Post-traumatic Hydrocephalus
- Perforation of the Urinary Bladder by the Distal Catheter: A Rare Complication of Ventriculoperitoneal Shunt
- Sigmoid Colon Perforation by a Distal Ventriculoperitoneal Shunt Catheter
- Shunt Complications
- Percutaneous Insertion of the Distal Catheter during Ventriculo-Atrial Shunts. A Simple and Reliable Method