J Korean Med Sci.
2021 Mar;36(9):e64. 10.3346/jkms.2021.36.e64.
Comparison of Serologic Response of Hospitalized COVID-19 Patients Using 8 Immunoassays
- Affiliations
-
- 1Department of Laboratory Medicine, Pusan National University Yangsan Hospital, Pusan National University School of Medicine, Yangsan, Korea
- 2Research Institute for Convergence of Biomedical Science and Technology, Pusan National University Yangsan Hospital, Yangsan, Korea
- 3Department of Laboratory Medicine, Pusan National University School of Medicine, Yangsan, Korea
- 4Division of Infection, Department of Internal Medicine, Pusan National University Yangsan Hospital, Yangsan, Korea
- 5Department of Laboratory Medicine, Pusan National University Hospital, Busan, Korea
- 6Department of Laboratory Medicine, Seoul Medical Center, Seoul, Korea
- KMID: 2513585
- DOI: http://doi.org/10.3346/jkms.2021.36.e64
Abstract
- Background
In Korea, there were issues regarding the use of immunoassays for anti-severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibodies to detect infection. So, we compared antibody results of eight kinds of commercial immunoassays using clinical remnant specimens.
Methods
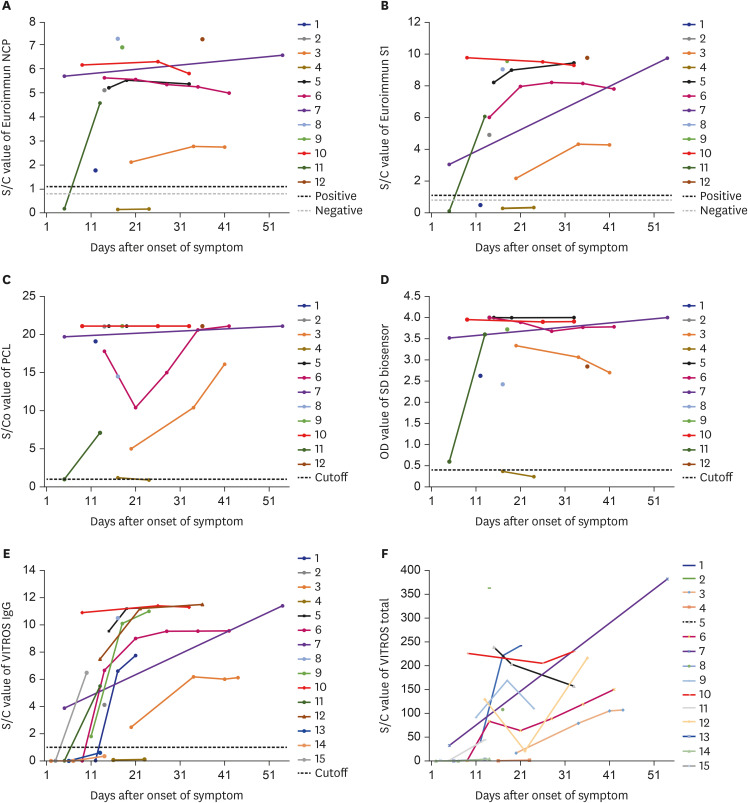
We compared the results of several immunoassay kits tested on 40 serum samples from 15 confirmed patients and 86 remnant serum samples from clinical laboratory. Eight kinds of IVD kits—four enzyme-linked immunosorbent assay, two lateral flow rapid immunochromatographic assays, and two chemiluminescent immunoassays with one RUO kit were tested.
Results
Among 40 serum samples from 15 coronavirus disease 2019 (COVID-19) patients, 35 yielded at least one positive result for detecting antibodies in the combined assessment. There were inconsistent results in 12 (28%) samples by single immunoassay. Forty samples collected in 2019 before the first COVID-19 Korean case showed negative results except for one equivocal result.
Conclusion
The discrepant results obtained with different immunoassay kits in this study show that serological assessment of SARS-CoV-2 by a single immunoassay requires caution not only in detecting infection but also in assessing immunologic status.
Keyword
Figure
Cited by 2 articles
-
Prevalence of SARS-CoV-2 Antibody in 2,935 Healthcare Workers at 6 Major Hospitals, Daegu, Korea
Yu Kyung Kim, Dohsik Minn, Do Young Song, Chae Hoon Lee, Nam Hee Ryoo, Chang-Ho Jeon, Kyung Eun Song, Jang Soo Suh, Soon Hee Chang
J Korean Med Sci. 2021;36(43):e294. doi: 10.3346/jkms.2021.36.e294.The Difference in Anti-nucleocapsid Protein Antibody Responses Between Vaccinated and Unvaccinated Individuals After Asymptomatic, Mild, or Moderate COVID-19 Infection
Ji Yeun Kim, Hye Hee Cha, Ji Soo Kwon, Jun Ho Cha, Choi Young Jang, Hyun Jung Lee, So Yun Lim, Sung-Han Kim
Korean J Healthc Assoc Infect Control Prev. 2023;28(1):92-98. doi: 10.14192/kjicp.2023.28.1.92.
Reference
-
1. World Health Organization. WHO coronavirus disease (COVID-19) dashboard. Updated 2020. Accessed October 26, 2020. https://covid19.who.int.2. Noh JY, Seo YB, Yoon JG, Seong H, Hyun H, Lee J, et al. Seroprevalence of anti-SARS-CoV-2 antibodies among outpatients in southwestern Seoul, Korea. J Korean Med Sci. 2020; 35(33):e311. PMID: 32830472.
Article3. Pickering S, Betancor G, Galão RP, Merrick B, Signell AW, Wilson HD, et al. Comparative assessment of multiple COVID-19 serological technologies supports continued evaluation of point-of-care lateral flow assays in hospital and community healthcare settings. PLoS Pathog. 2020; 16(9):e1008817. PMID: 32970782.
Article4. Padoan A, Cosma C, Sciacovelli L, Faggian D, Plebani M. Analytical performances of a chemiluminescence immunoassay for SARS-CoV-2 IgM/IgG and antibody kinetics. Clin Chem Lab Med. 2020; 58(7):1081–1088. PMID: 32301749.
Article5. Favresse J, Eucher C, Elsen M, Laffineur K, Dogné JM, Douxfils J. Response of anti-SARS-CoV-2 total antibodies to nucleocapsid antigen in COVID-19 patients: a longitudinal study. Clin Chem Lab Med. 2020; 58(10):e193–e196. PMID: 32639942.
Article6. Lee J, Kim SY, Sung H, Choe YJ, Hong KH. Letter to the editor: the interpretation of COVID-19 seroprevalence study should be cautious. J Korean Med Sci. 2020; 35(38):e338. PMID: 32989933.
Article7. Choi MH. Antibody test kits removed from U.S. FDA list of distributable items. Updated 2020. Accessed October 26, 2020. http://www.businesskorea.co.kr/news/articleView.html?idxno=50778.8. de Lusignan S, Lopez Bernal J, Zambon M, Akinyemi O, Amirthalingam G, Andrews N, et al. Emergence of a novel coronavirus (COVID-19): protocol for extending surveillance used by the Royal College of General Practitioners Research and Surveillance Centre and Public Health England. JMIR Public Health Surveill. 2020; 6(2):e18606. PMID: 32240095.
Article9. Lifshitz MS. Preanalysis. In : McPherson RA, Pincus MR, editors. Henry's Clinical Diagnosis and Management by Laboratory Methods. 23rd ed. Amsterdam: Elsevier Health Sciences;2017.10. Choe JY, Kim JW, Kwon HH, Hong HL, Jung CY, Jeon CH, et al. Diagnostic performance of immunochromatography assay for rapid detection of IgM and IgG in coronavirus disease 2019. J Med Virol. 2020; 92(11):2567–2572. PMID: 32458479.
Article11. Tang MS, Hock KG, Logsdon NM, Hayes JE, Gronowski AM, Anderson NW, et al. Clinical performance of two SARS-CoV-2 serologic assays. Clin Chem. 2020; 66(8):1055–1062. PMID: 32402061.
Article12. Jarrom D, Elston L, Washington J, Prettyjohns M, Cann K, Myles S, et al. Effectiveness of tests to detect the presence of SARS-CoV-2 virus, and antibodies to SARS-CoV-2, to inform COVID-19 diagnosis: a rapid systematic review. BMJ Evid Based Med. Forthcoming. 2020; DOI: 10.1136/bmjebm-2020-111511.
Article13. Ministry of Health and Welfare. Coronavirus disease-19, Republic of Korea. Updated 2020. Accessed October 30, 2020. http://ncov.mohw.go.kr.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Do Anxiety and Depression Levels Affect the Inflammation Response in Patients Hospitalized for COVID-19
- Post-COVID-19 Syndrome
- Impact of solid organ transplantation on the effectiveness of COVID-19 vaccination in hospitalized patients with COVID-19: a propensity score-matched cohort study
- New-Onset Seizures in Patients With COVID-19: A Case Series From a Single Public Hospital in Korea
- Vaccine hesitancy in patients with COVID-19 who have back pain