Korean J Gastroenterol.
2021 Feb;77(2):88-91. 10.4166/kjg.2020.145.
Retreatment of Chronic Hepatitis C Failed to Daclatasvir Plus Asunaprevir by Other Direct-acting Antivirals
- Affiliations
-
- 1Department of Internal Medicine, Seoul Paik Hospital, Inje University College of Medicine, Seoul, Korea
- KMID: 2513419
- DOI: http://doi.org/10.4166/kjg.2020.145
Abstract
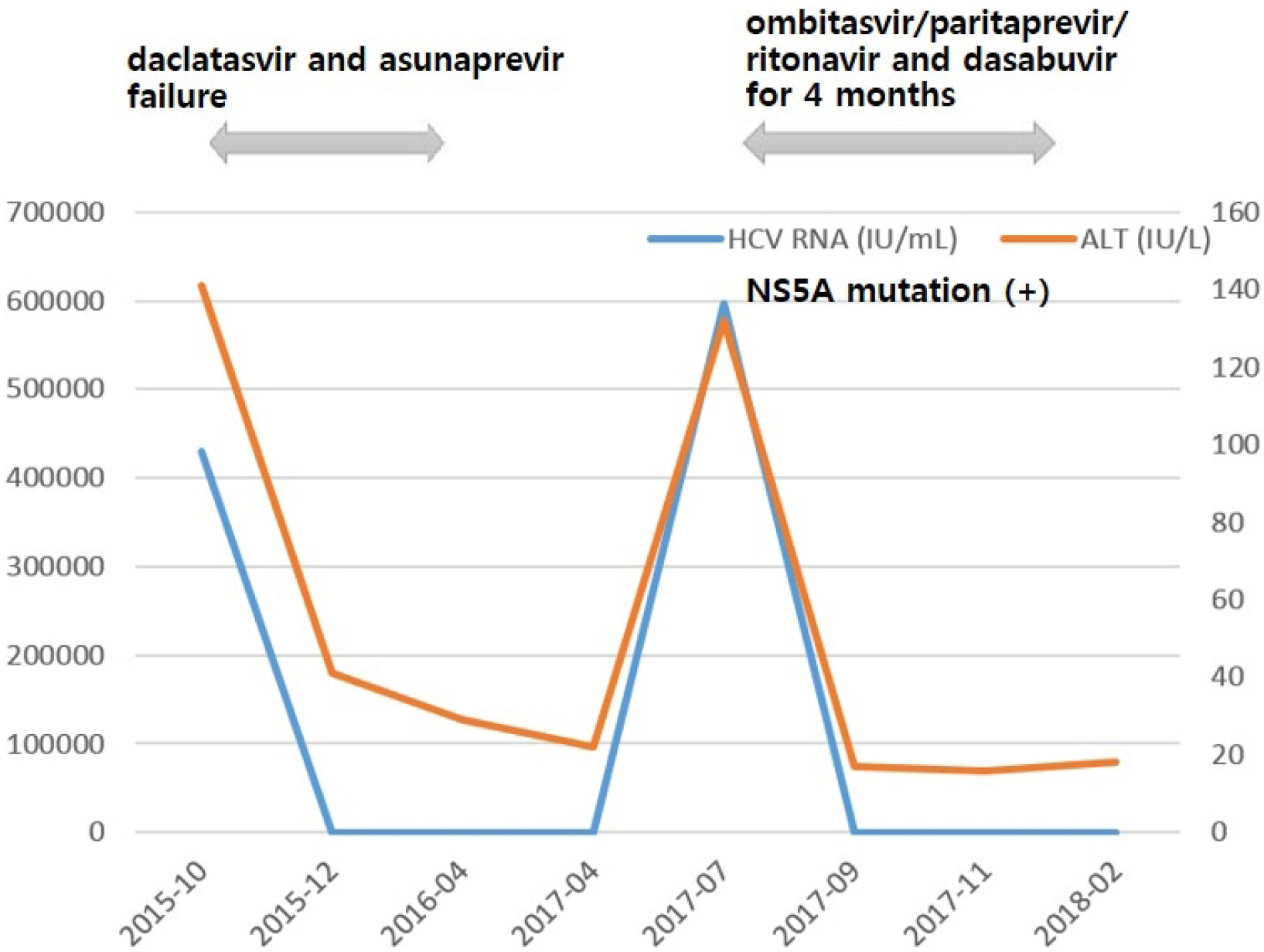
- The pegylated interferon plus ribavirin combination therapy has been used as the primary treatment for chronic hepatitis C (CHC) but fails to produce a sustained viral response (SVR) in many patients. In recent years, the treatment of CHC has been rapidly changing because of the introduction of direct-acting antivirals (DAAs), which have a high cure rate. However, retreatment of patients after failure of the first DAA therapy is difficult. We report two rare cases of CHC that showed acquired SVR with other DAA combinations after failure to daclatasvir and asunaprevir.
Keyword
Figure
Reference
-
1. Polaris Observatory HCV Collaborators. 2017; Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol. 2:161–176. DOI: 10.1016/S2468-1253(16)30181-9. PMID: 28404132.2. Feld JJ, Hoofnagle JH. 2005; Mechanism of action of interferon and ribavirin in treatment of hepatitis C. Nature. 436:967–972. DOI: 10.1038/nature04082. PMID: 16107837.
Article3. Manns MP, McHutchison JG, Gordon SC, et al. 2001; Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 358:958–965. DOI: 10.1016/S0140-6736(01)06102-5. PMID: 11583749.
Article4. Feld JJ, Foster GR. 2016; Second generation direct-acting antivirals - do we expect major improvements? J Hepatol. 65 Suppl 1:S130–S142. DOI: 10.1016/j.jhep.2016.07.007. PMID: 27641983.
Article5. Feld JJ, Kowdley KV, Coakley E, et al. 2014; Treatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med. 370:1594–1603. DOI: 10.1056/NEJMoa1315722. PMID: 24720703.
Article6. Poordad F, Hezode C, Trinh R, et al. 2014; ABT-450/r-ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosis. N Engl J Med. 370:1973–1982. DOI: 10.1056/NEJMoa1402869. PMID: 24725237.
Article7. AASLD/IDSA HCV Guidance Panel. 2015; Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology. 62:932–954. DOI: 10.1002/hep.27950. PMID: 26111063.8. European Association for the Study of the Liver. 2018; EASL recommendations on treatment of hepatitis C 2018. J Hepatol. 69:461–511. DOI: 10.1016/j.jhep.2018.03.026. PMID: 29650333.9. Forns X, Lee SS, Valdes J, et al. 2017; Glecaprevir plus pibrentasvir for chronic hepatitis C virus genotype 1, 2, 4, 5, or 6 infection in adults with compensated cirrhosis (EXPEDITION-1): a single-arm, open-label, multicentre phase 3 trial. Lancet Infect Dis. 17:1062–1068. DOI: 10.1016/S1473-3099(17)30496-6. PMID: 28818546.
Article10. Zeuzem S, Foster GR, Wang S, et al. 2018; Glecaprevir-pibrentasvir for 8 or 12 weeks in HCV genotype 1 or 3 infection. N Engl J Med. 378:354–369. DOI: 10.1056/NEJMoa1702417. PMID: 29365309.
Article11. Halfon P, Scholtès C, Izopet J, et al. 2018; Retreatment with direct-acting antivirals of genotypes 1-3-4 hepatitis C patients who failed an anti-NS5A regimen in real world. J Hepatol. 68:595–597. DOI: 10.1016/j.jhep.2017.09.019. PMID: 28987520.
Article12. Manns M, Pol S, Jacobson IM, et al. 2014; All-oral daclatasvir plus asunaprevir for hepatitis C virus genotype 1b: a multinational, phase 3, multicohort study. Lancet. 384:1597–1605. DOI: 10.1016/S0140-6736(14)61059-X. PMID: 25078304.
Article13. Korean Association for the Study of the Liver (KASL). 2018; 2017 KASL clinical practice guidelines management of hepatitis C: treatment of chronic hepatitis C. Clin Mol Hepatol. 24:169–229. DOI: 10.3350/cmh.2018.1004. PMID: 30092624. PMCID: PMC6166104.14. NS5A inhibitor DAA-experienced, genotype 1 patients. [Internet]. 2021. Jan. 21. American Associations for the Study of Liver Diseases (AASLD);Alexandria (VA): cited 2021 Jan 28. Available from https://www.hcvguidelines.org/treatment-experienced/multiple-daa-failure.15. Akuta N, Sezaki H, Suzuki F, et al. 2017; Ledipasvir plus sofosbuvir as salvage therapy for HCV genotype 1 failures to prior NS5A inhibitors regimens. J Med Virol. 89:1248–1254. DOI: 10.1002/jmv.24767. PMID: 28079269.
Article16. Zeuzem S, Jacobson IM, Baykal T, et al. 2014; Retreatment of HCV with ABT-450/r-ombitasvir and dasabuvir with ribavirin. N Engl J Med. 370:1604–1614. DOI: 10.1056/NEJMoa1401561. PMID: 24720679.
Article17. Krishnan P, Schnell G, Tripathi R, et al. 2015; Analysis of hepatitis C virus genotype 1b resistance variants in Japanese patients treated with paritaprevir-ritonavir and ombitasvir. Antimicrob Agents Chemother. 60:1106–1113. DOI: 10.1128/AAC.02606-15. PMID: 26643326. PMCID: PMC4750684.
Article18. Tojima H, Kakizaki S, Takakusagi S, et al. 2020; Favorable outcome of retreatment by direct-acting antivirals for hepatitis C patients with daclatasvir plus asunaprevir combination therapy failure. Hepatol Res. 50:303–312. DOI: 10.1111/hepr.13462. PMID: 31750974.19. Kumada H, Watanabe T, Suzuki F, et al. 2018; Efficacy and safety of glecaprevir/pibrentasvir in HCV-infected Japanese patients with prior DAA experience, severe renal impairment, or genotype 3 infection. J Gastroenterol. 53:566–575. DOI: 10.1007/s00535-017-1396-0. PMID: 29052790. PMCID: PMC5866827.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- 2017 KASL clinical practice guidelines management of hepatitis C: Treatment of chronic hepatitis C
- New direct-acting antivirals for the treatment of chronic hepatitis C
- Direct Acting Antiviral Agents in Korean Patients with Chronic Hepatitis C and Hemophilia Who Are Treatment-Naïve or Treatment-Experienced
- Efficacy and safety of daclatasvir and asunaprevir for hepatitis C virus genotype 1b infection
- Next-generation sequencing analysis of hepatitis C virus resistance–associated substitutions in direct-acting antiviral failure in South Korea