Endocrinol Metab.
2021 Feb;36(1):157-170. 10.3803/EnM.2020.781.
Effects of Glucagon-Like Peptide-1 Analogue and Fibroblast Growth Factor 21 Combination on the Atherosclerosis-Related Process in a Type 2 Diabetes Mouse Model
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea
- 2Department of Internal Medicine, Korea University Anam Hospital, Korea University College of Medicine, Seoul, Korea
- KMID: 2513299
- DOI: http://doi.org/10.3803/EnM.2020.781
Abstract
- Background
Glucagon-like peptide-1 (GLP-1) analogues regulate glucose homeostasis and have anti-inflammatory properties, but cause gastrointestinal side effects. The fibroblast growth factor 21 (FGF21) is a hormonal regulator of lipid and glucose metabolism that has poor pharmacokinetic properties, including a short half-life. To overcome these limitations, we investigated the effect of a low-dose combination of a GLP-1 analogue and FGF21 on atherosclerosis-related molecular pathways.
Methods
C57BL/6J mice were fed a high-fat diet for 30 weeks followed by an atherogenic diet for 10 weeks and were divided into four groups: control (saline), liraglutide (0.3 mg/kg/day), FGF21 (5 mg/kg/day), and low-dose combination treatment with liraglutide (0.1 mg/kg/day) and FGF21 (2.5 mg/kg/day) (n=6/group) for 6 weeks. The effects of each treatment on various atherogenesisrelated pathways were assessed.
Results
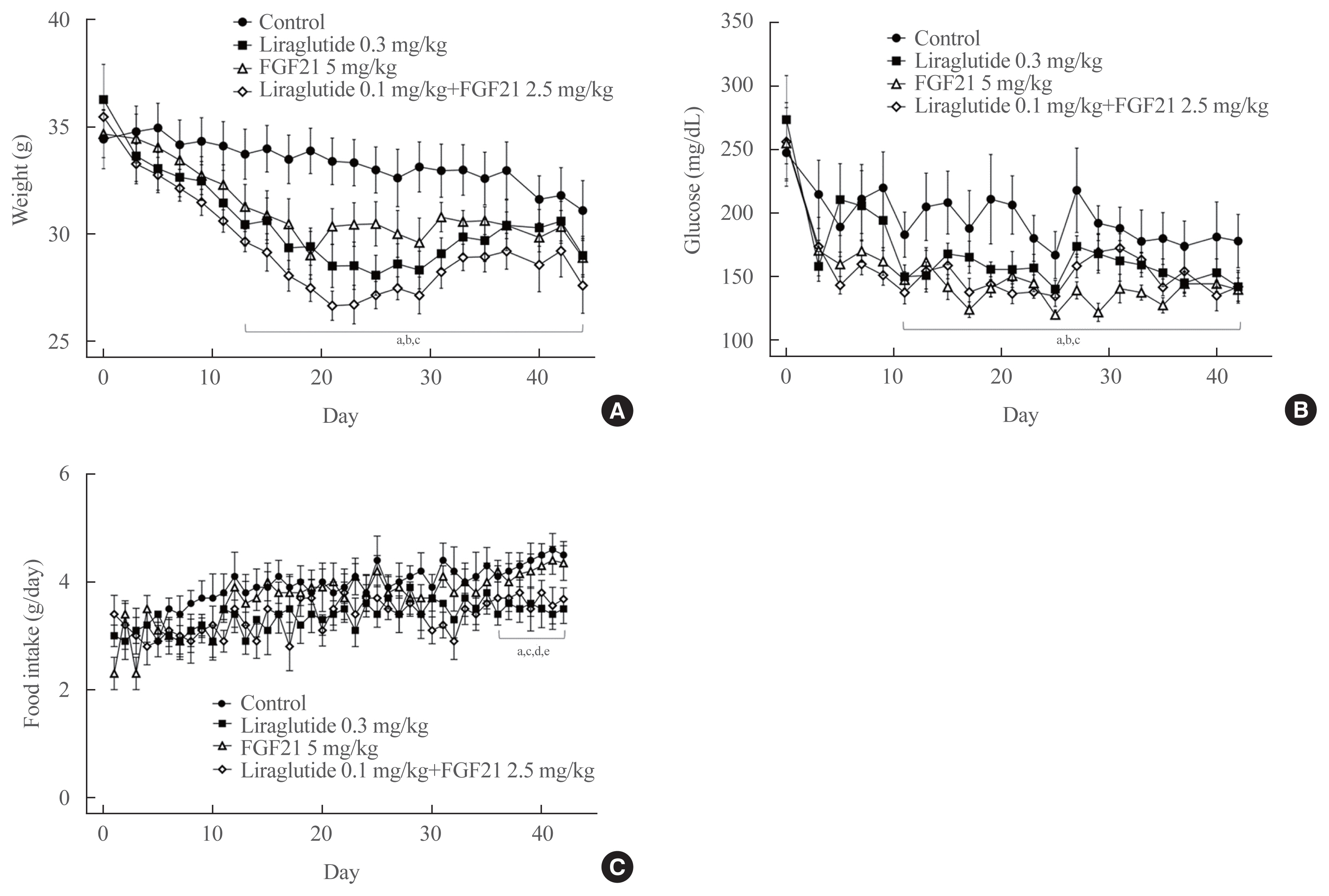
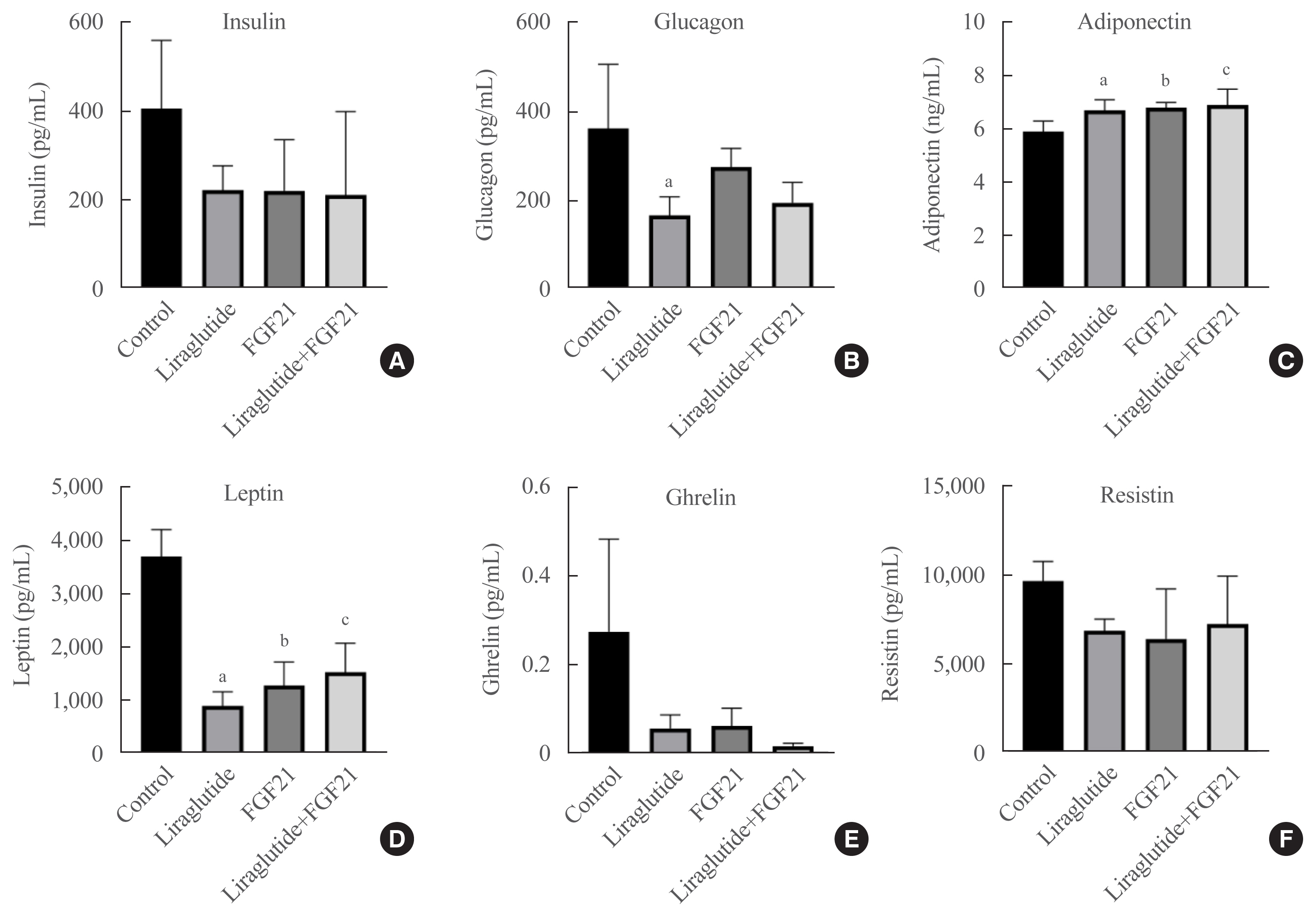
Liraglutide, FGF21, and their low-dose combination significantly reduced atheromatous plaque in aorta, decreased weight, glucose, and leptin levels, and increased adiponectin levels. The combination treatment upregulated the hepatic uncoupling protein-1 (UCP1) and Akt1 mRNAs compared with controls. Matric mentalloproteinase-9 (MMP-9), monocyte chemoattractant protein-1 (MCP-1), and intercellular adhesion molecule-1 (ICAM-1) were downregulated and phosphorylated Akt (p-Akt) and phosphorylated extracellular signal-regulated kinase (p-ERK) were upregulated in liver of the liraglutide-alone and combination-treatment groups. The combination therapy also significantly decreased the proliferation of vascular smooth muscle cells. Caspase-3 was increased, whereas MMP-9, ICAM-1, p-Akt, and p-ERK1/2 were downregulated in the liraglutide-alone and combination-treatment groups.
Conclusion
Administration of a low-dose GLP-1 analogue and FGF21 combination exerts beneficial effects on critical pathways related to atherosclerosis, suggesting the synergism of the two compounds.
Keyword
Figure
Cited by 1 articles
-
Unlocking the Therapeutic Potential of Glucagon-Like Peptide-1 Analogue and Fibroblast Growth Factor 21 Combination for the Pathogenesis of Atherosclerosis in Type 2 Diabetes
Jang Won Son
Endocrinol Metab. 2021;36(1):57-59. doi: 10.3803/EnM.2021.109.
Reference
-
1. Martin-Timon I, Sevillano-Collantes C, Segura-Galindo A, Del Canizo-Gomez FJ. Type 2 diabetes and cardiovascular disease: have all risk factors the same strength? World J Diabetes. 2014; 5:444–70.
Article2. Abdul-Ghani M, DeFronzo RA, Del Prato S, Chilton R, Singh R, Ryder REJ. Cardiovascular disease and type 2 diabetes: has the dawn of a new era arrived? Diabetes Care. 2017; 40:813–20.
Article3. Aronson D, Bloomgarden Z, Rayfield EJ. Potential mechanisms promoting restenosis in diabetic patients. J Am Coll Cardiol. 1996; 27:528–35.
Article4. Ben-Shlomo S, Zvibel I, Shnell M, Shlomai A, Chepurko E, Halpern Z, et al. Glucagon-like peptide-1 reduces hepatic lipogenesis via activation of AMP-activated protein kinase. J Hepatol. 2011; 54:1214–23.
Article5. Chai W, Dong Z, Wang N, Wang W, Tao L, Cao W, et al. Glucagon-like peptide 1 recruits microvasculature and increases glucose use in muscle via a nitric oxide-dependent mechanism. Diabetes. 2012; 61:888–96.
Article6. Gao H, Wang X, Zhang Z, Yang Y, Yang J, Li X, et al. GLP-1 amplifies insulin signaling by up-regulation of IRbeta, IRS-1 and Glut4 in 3T3-L1 adipocytes. Endocrine. 2007; 32:90–5.7. Lee YS, Park MS, Choung JS, Kim SS, Oh HH, Choi CS, et al. Glucagon-like peptide-1 inhibits adipose tissue macrophage infiltration and inflammation in an obese mouse model of diabetes. Diabetologia. 2012; 55:2456–68.
Article8. Lim S, Kim KM, Nauck MA. Glucagon-like peptide-1 receptor agonists and cardiovascular events: class effects versus individual patterns. Trends Endocrinol Metab. 2018; 29:238–48.
Article9. Holman RR, Bethel MA, Mentz RJ, Thompson VP, Lokhnygina Y, Buse JB, et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2017; 377:1228–39.
Article10. Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jodar E, Leiter LA, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016; 375:1834–44.
Article11. Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016; 375:311–22.
Article12. Pfeffer MA, Claggett B, Diaz R, Dickstein K, Gerstein HC, Kober LV, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015; 373:2247–57.
Article13. Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomized placebo-controlled trial. Lancet. 2019; 394:121–30.14. Ard J, Cannon A, Lewis CE, Lofton H, Vang Skjoth T, Stevenin B, et al. Efficacy and safety of liraglutide 3.0 mg for weight management are similar across races: subgroup analysis across the SCALE and phase II randomized trials. Diabetes Obes Metab. 2016; 18:430–5.
Article15. Itoh N, Ornitz DM. Evolution of the Fgf and Fgfr gene families. Trends Genet. 2004; 20:563–9.
Article16. Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ, et al. FGF-21 as a novel metabolic regulator. J Clin Invest. 2005; 115:1627–35.
Article17. Nishimura T, Nakatake Y, Konishi M, Itoh N. Identification of a novel FGF, FGF-21, preferentially expressed in the liver. Biochim Biophys Acta. 2000; 1492:203–6.
Article18. Kharitonenkov A, Wroblewski VJ, Koester A, Chen YF, Clutinger CK, Tigno XT, et al. The metabolic state of diabetic monkeys is regulated by fibroblast growth factor-21. Endocrinology. 2007; 148:774–81.
Article19. Xu J, Lloyd DJ, Hale C, Stanislaus S, Chen M, Sivits G, et al. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes. 2009; 58:250–9.
Article20. Lin Z, Pan X, Wu F, Ye D, Zhang Y, Wang Y, et al. Fibroblast growth factor 21 prevents atherosclerosis by suppression of hepatic sterol regulatory element-binding protein-2 and induction of adiponectin in mice. Circulation. 2015; 131:1861–71.
Article21. Inagaki T, Dutchak P, Zhao G, Ding X, Gautron L, Parameswara V, et al. Endocrine regulation of the fasting response by PPARalpha-mediated induction of fibroblast growth factor 21. Cell Metab. 2007; 5:415–25.22. So WY, Leung PS. Fibroblast growth factor 21 as an emerging therapeutic target for type 2 diabetes mellitus. Med Res Rev. 2016; 36:672–704.
Article23. Han JH, Oh TJ, Lee G, Maeng HJ, Lee DH, Kim KM, et al. The beneficial effects of empagliflozin, an SGLT2 inhibitor, on atherosclerosis in ApoE (−/−) mice fed a western diet. Diabetologia. 2017; 60:364–76.
Article24. Neuhoff S, Moers J, Rieks M, Grunwald T, Jensen A, Dermietzel R, et al. Proliferation, differentiation, and cytokine secretion of human umbilical cord blood-derived mononuclear cells in vitro. Exp Hematol. 2007; 35:1119–31.
Article25. Hur KY, Seo HJ, Kang ES, Kim SH, Song S, Kim EH, et al. Therapeutic effect of magnesium lithospermate B on neointimal formation after balloon-induced vascular injury. Eur J Pharmacol. 2008; 586:226–33.
Article26. Chapnik N, Genzer Y, Froy O. Relationship between FGF21 and UCP1 levels under time-restricted feeding and high-fat diet. J Nutr Biochem. 2017; 40:116–21.
Article27. Decara J, Arrabal S, Beiroa D, Rivera P, Vargas A, Serrano A, et al. Antiobesity efficacy of GLP-1 receptor agonist liraglutide is associated with peripheral tissue-specific modulation of lipid metabolic regulators. Biofactors. 2016; 42:600–11.
Article28. Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004; 84:277–359.
Article29. Liu P, Yang J, Chen ZY, Zhang P, Shi GJ. Mitochondrial protein UCP1 mediates liver injury induced by LPS through EKR signaling pathway. Eur Rev Med Pharmacol Sci. 2017; 21:3674–9.30. Ono H, Shimano H, Katagiri H, Yahagi N, Sakoda H, Onishi Y, et al. Hepatic Akt activation induces marked hypoglycemia, hepatomegaly, and hypertriglyceridemia with sterol regulatory element binding protein involvement. Diabetes. 2003; 52:2905–13.
Article31. Zhou J, Poudel A, Chandramani-Shivalingappa P, Xu B, Welchko R, Li L. Liraglutide induces beige fat development and promotes mitochondrial function in diet induced obesity mice partially through AMPK-SIRT-1-PGC1-α cell signaling pathway. Endocrine. 2019; 64:271–83.
Article32. Samms RJ, Smith DP, Cheng CC, Antonellis PP, Perfield JW 2nd, Kharitonenkov A, et al. Discrete aspects of FGF21 in vivo pharmacology do not require UCP1. Cell Rep. 2015; 11:991–9.33. Osaka T, Endo M, Yamakawa M, Inoue S. Energy expenditure by intravenous administration of glucagon-like peptide-1 mediated by the lower brainstem and sympathoadrenal system. Peptides. 2005; 26:1623–31.
Article34. Lockie SH, Heppner KM, Chaudhary N, Chabenne JR, Morgan DA, Veyrat-Durebex C, et al. Direct control of brown adipose tissue thermogenesis by central nervous system glucagon-like peptide-1 receptor signaling. Diabetes. 2012; 61:2753–62.
Article35. Achari AE, Jain SK. Adiponectin, a therapeutic target for obesity, diabetes, and endothelial dysfunction. Int J Mol Sci. 2017; 18:1321.
Article36. Hui X, Lam KS, Vanhoutte PM, Xu A. Adiponectin and cardiovascular health: an update. Br J Pharmacol. 2012; 165:574–90.
Article37. Cui H, Lopez M, Rahmouni K. The cellular and molecular bases of leptin and ghrelin resistance in obesity. Nat Rev Endocrinol. 2017; 13:338–51.
Article38. Frederich RC, Hamann A, Anderson S, Lollmann B, Lowell BB, Flier JS. Leptin levels reflect body lipid content in mice: evidence for diet-induced resistance to leptin action. Nat Med. 1995; 1:1311–4.
Article39. Lim S, Lee GY, Park HS, Lee DH, Oh TJ, Kim KM, et al. Attenuation of carotid neointimal formation after direct delivery of a recombinant adenovirus expressing glucagon-like peptide-1 in diabetic rats. Cardiovasc Res. 2017; 113:183–94.
Article40. Jojima T, Uchida K, Akimoto K, Tomotsune T, Yanagi K, Iijima T, et al. Liraglutide, a GLP-1 receptor agonist, inhibits vascular smooth muscle cell proliferation by enhancing AMP-activated protein kinase and cell cycle regulation, and delays atherosclerosis in ApoE deficient mice. Atherosclerosis. 2017; 261:44–51.
Article41. Jawien A, Bowen-Pope DF, Lindner V, Schwartz SM, Clowes AW. Platelet-derived growth factor promotes smooth muscle migration and intimal thickening in a rat model of balloon angioplasty. J Clin Invest. 1992; 89:507–11.
Article42. Bennett MR, Evan GI, Schwartz SM. Apoptosis of human vascular smooth muscle cells derived from normal vessels and coronary atherosclerotic plaques. J Clin Invest. 1995; 95:2266–74.
Article43. Kardas G, Daszynska-Kardas A, Marynowski M, Brzakalska O, Kuna P, Panek M. Role of platelet-derived growth factor (PDGF) in asthma as an immunoregulatory factor mediating airway remodeling and possible pharmacological target. Front Pharmacol. 2020; 11:47.
Article44. Perlman H, Sata M, Krasinski K, Dorai T, Buttyan R, Walsh K. Adenovirus-encoded hammerhead ribozyme to Bcl-2 inhibits neointimal hyperplasia and induces vascular smooth muscle cell apoptosis. Cardiovasc Res. 2000; 45:570–8.
Article45. Clarke MC, Figg N, Maguire JJ, Davenport AP, Goddard M, Littlewood TD, et al. Apoptosis of vascular smooth muscle cells induces features of plaque vulnerability in atherosclerosis. Nat Med. 2006; 12:1075–80.
Article46. Lim S, Lee KS, Lee JE, Park HS, Kim KM, Moon JH, et al. Effect of a new PPAR-gamma agonist, lobeglitazone, on neointimal formation after balloon injury in rats and the development of atherosclerosis. Atherosclerosis. 2015; 243:107–19.
Article47. Rolfe BE, Muddiman JD, Smith NJ, Campbell GR, Campbell JH. ICAM-1 expression by vascular smooth muscle cells is phenotype-dependent. Atherosclerosis. 2000; 149:99–110.
Article48. Newby AC. Dual role of matrix metalloproteinases (matrixins) in intimal thickening and atherosclerotic plaque rupture. Physiol Rev. 2005; 85:1–31.
Article49. Jiang Y, Beller DI, Frendl G, Graves DT. Monocyte chemoattractant protein-1 regulates adhesion molecule expression and cytokine production in human monocytes. J Immunol. 1992; 148:2423–8.50. Newby AC, Zaltsman AB. Molecular mechanisms in intimal hyperplasia. J Pathol. 2000; 190:300–9.
Article51. Senior RM, Griffin GL, Fliszar CJ, Shapiro SD, Goldberg GI, Welgus HG. Human 92- and 72-kilodalton type IV collagenases are elastases. J Biol Chem. 1991; 266:7870–5.
Article52. Lin J, Kakkar V, Lu X. Impact of MCP-1 in atherosclerosis. Curr Pharm Des. 2014; 20:4580–8.53. Lee YS, Jun HS. Anti-inflammatory effects of glp-1-based therapies beyond glucose control. Mediators Inflamm. 2016; 2016:3094642.
Article54. Jia H, Cheng J, Zhou Q, Peng J, Pan Y, Han H. Fibroblast growth factor 21 attenuates inflammation and oxidative stress in atherosclerotic rat via enhancing the Nrf1-ARE signaling pathway. Int J Clin Exp Pathol. 2018; 11:1308–17.55. Bao L, Yin J, Gao W, Wang Q, Yao W, Gao X. A long-acting FGF21 alleviates hepatic steatosis and inflammation in a mouse model of non-alcoholic steatohepatitis partly through an FGF21-adiponectin-IL17A pathway. Br J Pharmacol. 2018; 175:3379–93.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Unlocking the Therapeutic Potential of Glucagon-Like Peptide-1 Analogue and Fibroblast Growth Factor 21 Combination for the Pathogenesis of Atherosclerosis in Type 2 Diabetes
- Glucagon-Like Peptide-1 (GLP-1) Agonist
- Glucagon-like Peptide-1 Analogue and Dipeptidyl Peptidase-IV Inhibitors
- Glucagon-like peptide-1 and glucagon-like peptide-1 receptor agonists in the treatment of type 2 diabetes
- Glucagon-Like Peptide-1 Receptor Agonists for the Treatment of Type 2 Diabetes Mellitus: A Position Statement of the Korean Diabetes Association