Int J Stem Cells.
2021 Feb;14(1):58-73. 10.15283/ijsc19121.
TL1A/TNFR2 Axis Enhances Immunoregulatory Effects of Bone Marrow Derived Mesenchymal Stem Cell by Indian Hedgehog Signaling Pathway
- Affiliations
-
- 1Department of Immunology, College of Basic Medical Science, Dalian Medical University, Liaoning, China
- 2Department of Immunology, Guangzhou Institute of Pediatrics, Guangzhou Women and Children’s Medical Center, Guangzhou Medical University, Guangzhou, China
- 3Molecular Medicine Laboratory, College of Basic Medical Science, Dalian Medical University, Liaoning, China
- 4Department of Pharmacology, College of Pharmacy, Dalian Medical University, Liaoning, China
- 5Microbiology Laboratory, College of Basic Medical Science, Dalian Medical University, Liaoning, China
- 6Key Laboratory of Molecular Epigenetics of MOE, School of Life Science, Northeast Normal University, Changchun, China
- 7Department of Clinical Microbiology, School of Medicine and Health Sciences, University for Development Studies, Tamale, Ghana
- KMID: 2513083
- DOI: http://doi.org/10.15283/ijsc19121
Abstract
- Background and Objectives
The immunomodulatory potential of mesenchymal stem cells (MSCs) can be regulated by a variety of molecules, especially cytokines. The inflammatory cytokine, TNF-like ligand 1A (TL1A), has been reported as an inflammation stimulator in-multiple autoimmune diseases. Here, we studied the effects of TL1A/TNF-receptor 2 (TNFR2) pathway on the therapeutic potency of bone marrow-derived MSCs (BMSCs).
Methods and Results
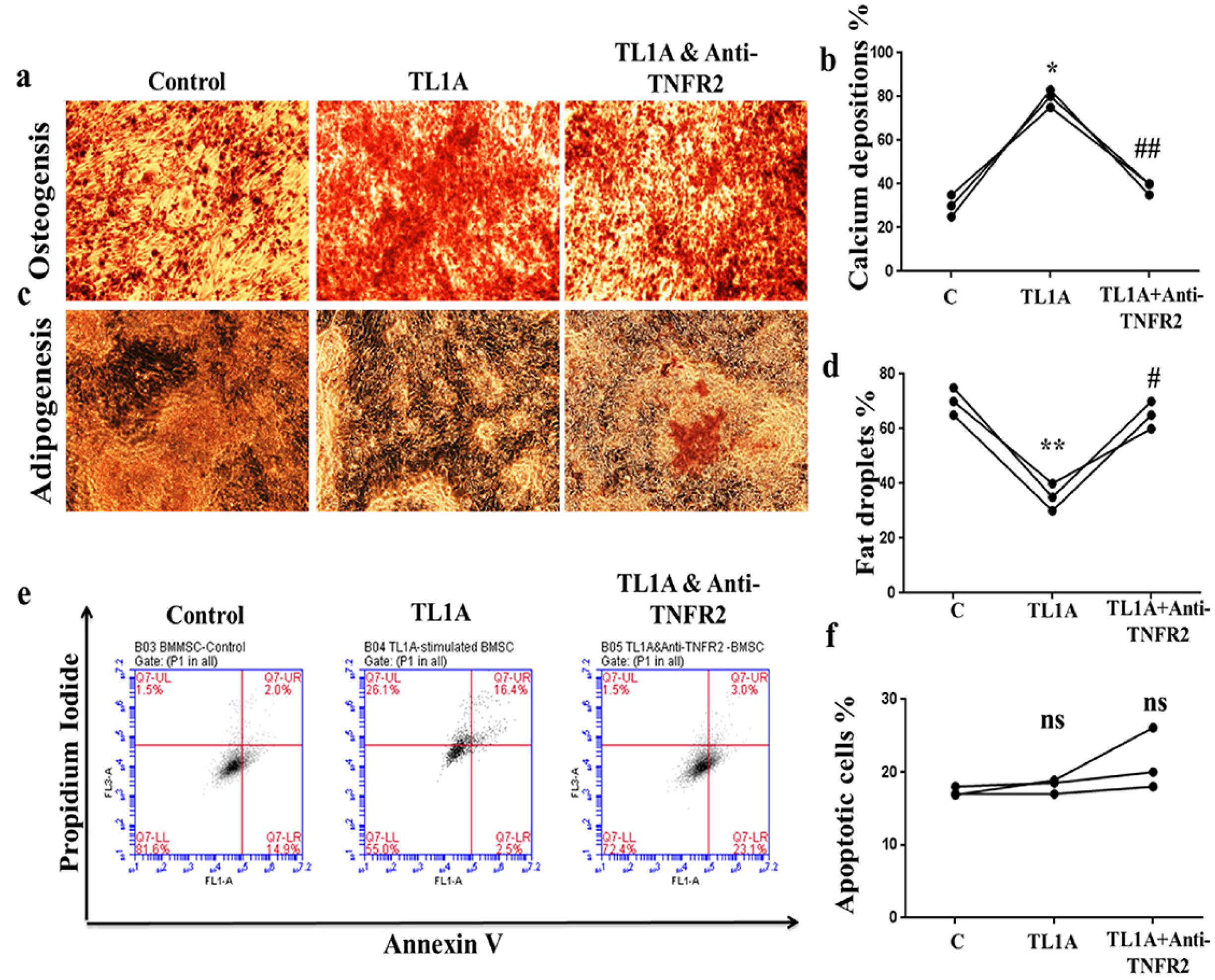
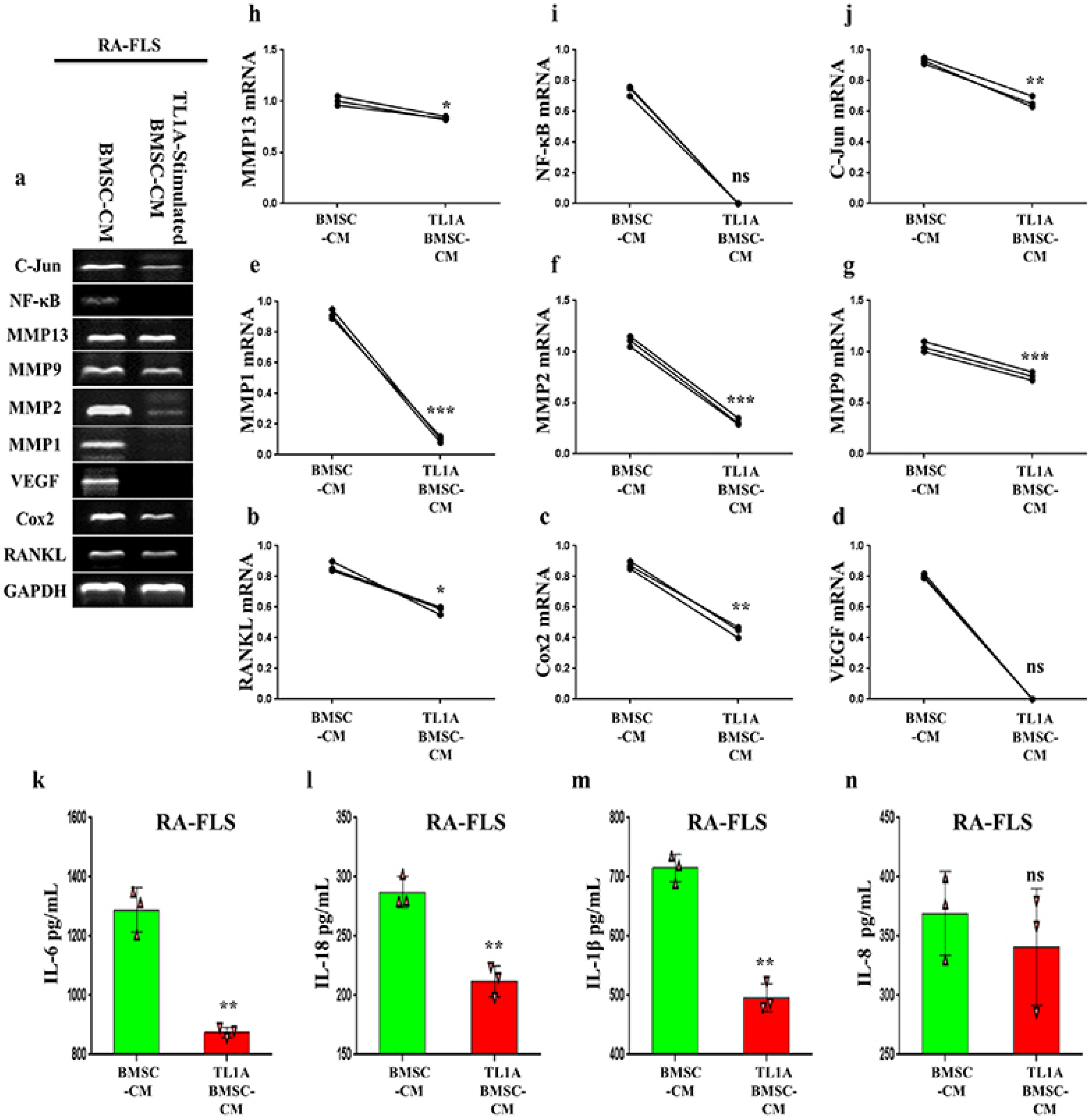
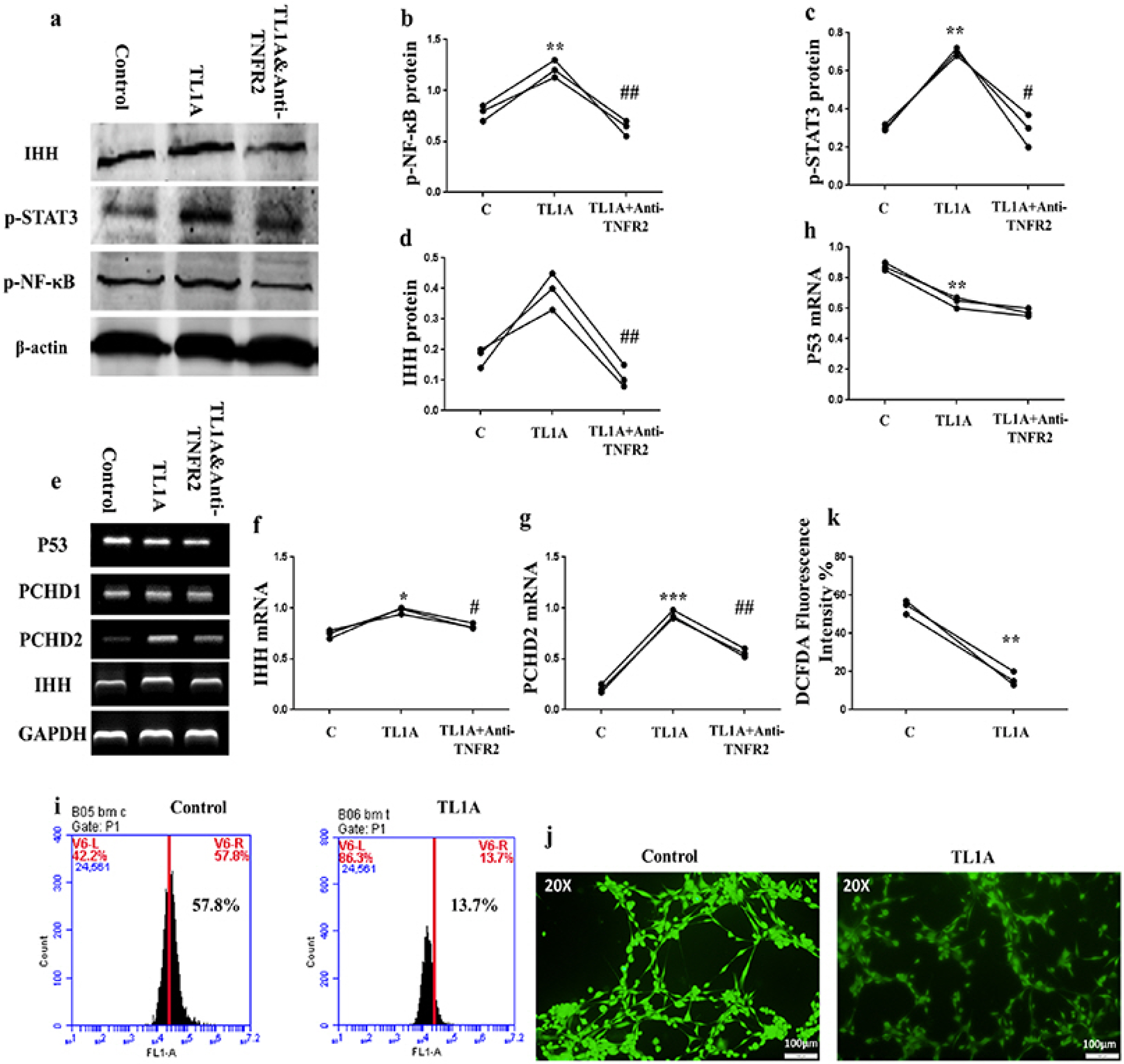
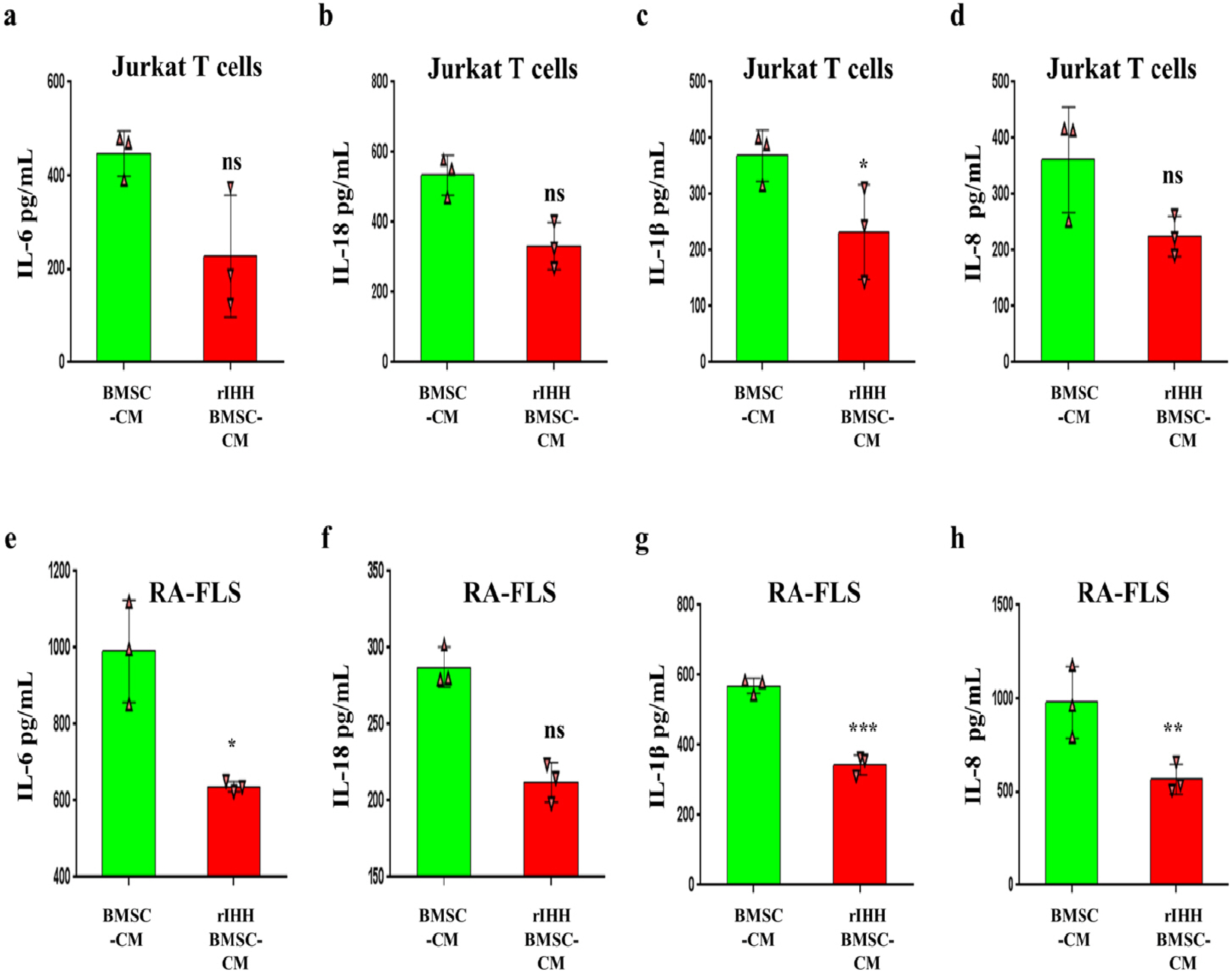
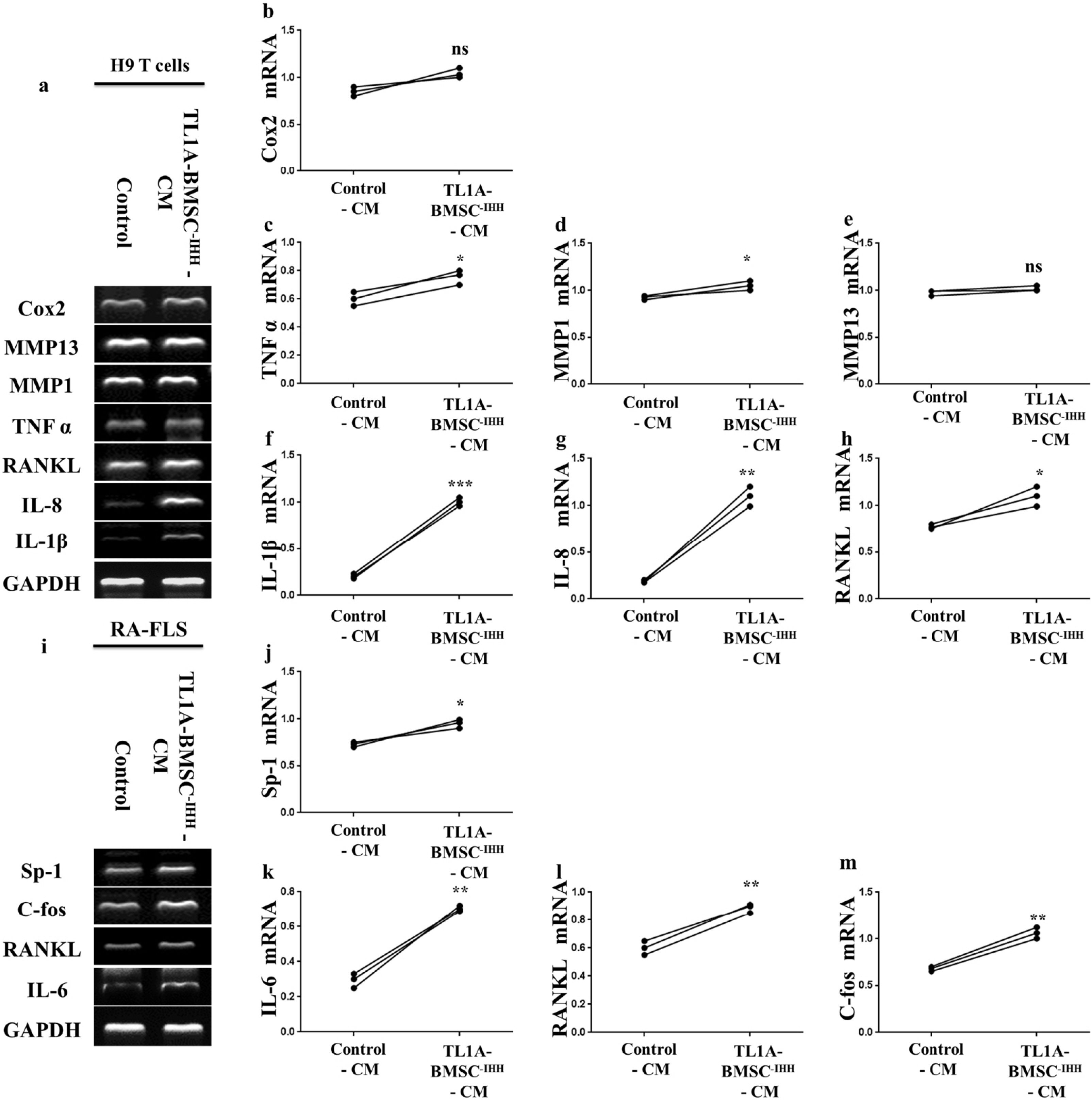
BMSCs, fibroblast-like synoviocytes (FLSs), and H9 and jurkat human T lymphocytes were used in this study. BMSCs paracrine activities, differentiation, proliferation, and migration were investigated after stimulation with TL1A, and intervened with anti-TNFR2. Additionally, the effects of TL1A on BMSCs therapeutic potency were evaluated by treating RA-FLSs, and H9 and jurkat T cells with TL1A-stimulated BMSCs conditioned medium (CM). Indian hedgehog (IHH) involvement was determined by gene silencing and treatment by recombinant IHH (rIHH). TL1A induced BMSCs stemness-related genes, COX-2, IL-6, IDO, TGF-β and HGF through TNFR2. Also, TL1A corrected biased differentiation and increased proliferation, and migration through TNFR2. Meanwhile, CM of TL1A-stimulated BMSCs decreased the inflammatory markers of RA-FLSs and T cells. Moreover, TL1A-stimulated BMSCs experienced IHH up-regulation coupled with NF-κB and STAT3 signaling up-regulation, while p53 and oxidative stress were down-regulated. Furthermore, treatment of BMSCs by rIHH increased their anti-inflammatory effects. More importantly, knockdown of IHH decreased the ability of TL1A-stimulated BMSCs to alleviating the inflammation in RA-FLSs and T cells.
Conclusions
This study reports the effects of TL1A/TNFR2 pathway on the biological behaviors and therapeutic potency of BMSCs through IHH. These findings could introduce novel procedures to increase the stemness of MSCs in cellular therapy.
Keyword
Figure
Reference
-
References
1. Friedenstein AJ, Gorskaja JF, Kulagina NN. 1976; Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol. 4:267–274. PMID: 976387.2. Prockop DJ. 1997; Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 276:71–74. DOI: 10.1126/science.276.5309.71. PMID: 9082988.
Article3. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. 1999; Multilineage potential of adult human mesenchymal stem cells. Science. 284:143–147. DOI: 10.1126/science.284.5411.143. PMID: 10102814.
Article4. Smith JR, Pochampally R, Perry A, Hsu SC, Prockop DJ. 2004; Isolation of a highly clonogenic and multipotential subfraction of adult stem cells from bone marrow stroma. Stem Cells. 22:823–831. DOI: 10.1634/stemcells.22-5-823. PMID: 15342946.
Article5. Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, Du J, Aldrich S, Lisberg A, Low WC, Largaespada DA, Verfaillie CM. 2002; Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 418:41–49. DOI: 10.1038/nature00870. PMID: 12077603.
Article6. Krampera M, Franchini M, Pizzolo G, Aprili G. 2007; Mesenchymal stem cells: from biology to clinical use. Blood Transfus. 5:120–129. DOI: 10.2450/2007.0029-07. PMID: 19204764. PMCID: PMC2535891.7. Nagamura-Inoue T, Mukai T. 2016; Umbilical cord is a rich source of mesenchymal stromal cells for cell therapy. Curr Stem Cell Res Ther. 11:634–642. DOI: 10.2174/1574888X10666151026115017. PMID: 26496885.
Article8. Koç ON, Gerson SL, Cooper BW, Dyhouse SM, Haynesworth SE, Caplan AI, Lazarus HM. 2000; Rapid hematopoietic recovery after coinfusion of autologous-blood stem cells and culture- expanded marrow mesenchymal stem cells in advanced breast cancer patients receiving high-dose chemotherapy. J Clin Oncol. 18:307–316. DOI: 10.1200/JCO.2000.18.2.307. PMID: 10637244.9. Burks SR, Nagle ME, Bresler MN, Kim SJ, Star RA, Frank JA. 2018; Mesenchymal stromal cell potency to treat acute kidney injury increased by ultrasound-activated interferon-γ/in-terleukin-10 axis. J Cell Mol Med. 22:6015–6025. DOI: 10.1111/jcmm.13874. PMID: 30216653. PMCID: PMC6237567.
Article10. Gurung S, Williams S, Deane JA, Werkmeister JA, Gargett CE. 2018; The transcriptome of human endometrial mesenchymal stem cells under TGFβR inhibition reveals improved potential for cell-based therapies. Front Cell Dev Biol. 6:164. DOI: 10.3389/fcell.2018.00164. PMID: 30564575. PMCID: PMC6288489.
Article11. Le Blanc K, Tammik L, Sundberg B, Haynesworth SE, Ringdén O. 2003; Mesenchymal stem cells inhibit and stimulate mixed lymphocyte cultures and mitogenic responses independently of the major histocompatibility complex. Scand J Immunol. 57:11–20. DOI: 10.1046/j.1365-3083.2003.01176.x. PMID: 12542793.
Article12. Migone TS, Zhang J, Luo X, Zhuang L, Chen C, Hu B, Hong JS, Perry JW, Chen SF, Zhou JX, Cho YH, Ullrich S, Kanakaraj P, Carrell J, Boyd E, Olsen HS, Hu G, Pukac L, Liu D, Ni J, Kim S, Gentz R, Feng P, Moore PA, Ruben SM, Wei P. 2002; TL1A is a TNF-like ligand for DR3 and TR6/DcR3 and functions as a T cell costimulator. Immunity. 16:479–492. DOI: 10.1016/S1074-7613(02)00283-2. PMID: 11911831.
Article13. Meylan F, Davidson TS, Kahle E, Kinder M, Acharya K, Jankovic D, Bundoc V, Hodges M, Shevach EM, Keane-Myers A, Wang EC, Siegel RM. 2008; The TNF-family receptor DR3 is essential for diverse T cell-mediated inflammatory diseases. Immunity. 29:79–89. DOI: 10.1016/j.immuni.2008.04.021. PMID: 18571443. PMCID: PMC2760084.
Article14. Ma Z, Wang B, Wang M, Sun X, Tang Y, Li M, Li F, Li X. 2016; TL1A increased IL-6 production on fibroblast-like synoviocytes by preferentially activating TNF receptor 2 in rheumatoid arthritis. Cytokine. 83:92–98. DOI: 10.1016/j.cyto.2016.04.005. PMID: 27081759.
Article15. Siakavellas SI, Bamias G. 2015; Tumor necrosis factor-like cytokine TL1A and its receptors DR3 and DcR3: important new factors in mucosal homeostasis and inflammation. Inflamm Bowel Dis. 21:2441–2452. DOI: 10.1097/MIB.0000000000000492. PMID: 26099067.16. Matsumura E, Tsuji K, Komori K, Koga H, Sekiya I, Muneta T. 2017; Pretreatment with IL-1β enhances proliferation and chondrogenic potential of synovium-derived mesenchymal stem cells. Cytotherapy. 19:181–193. DOI: 10.1016/j.jcyt.2016.11.004. PMID: 27979606.
Article17. Chen MS, Lin CY, Chiu YH, Chen CP, Tsai PJ, Wang HS. 2018; IL-1β-induced matrix metalloprotease-1 promotes mesenchymal stem cell migration via PAR1 and G-protein-cou-pled signaling pathway. Stem Cells Int. 2018:3524759. DOI: 10.1155/2018/3524759. PMID: 30026761. PMCID: PMC6031215.
Article18. Putra A, Ridwan FB, Putridewi AI, Kustiyah AR, Wirastuti K, Sadyah NAC, Rosdiana I, Munir D. 2018; The role of TNF-α induced MSCs on suppressive inflammation by increasing TGF-β and IL-10. Open Access Maced J Med Sci. 6:1779–1783. DOI: 10.3889/oamjms.2018.404. PMID: 30455748. PMCID: PMC6236029.
Article19. Katoh Y, Katoh M. 2006; WNT antagonist, SFRP1, is Hedgehog signaling target. Int J Mol Med. 17:171–175. DOI: 10.3892/ijmm.17.1.171. PMID: 16328026.
Article20. Ingham PW, McMahon AP. 2001; Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 15:3059–3087. DOI: 10.1101/gad.938601. PMID: 11731473.
Article21. Kawagishi H, Xiong J, Rovira II, Pan H, Yan Y, Fleischmann BK, Yamada M, Finkel T. 2018; Sonic hedgehog signaling regulates the mammalian cardiac regenerative response. J Mol Cell Cardiol. 123:180–184. DOI: 10.1016/j.yjmcc.2018.09.005. PMID: 30236923.
Article22. Al-Azab M, Wang B, Elkhider A, Walana W, Li W, Yuan B, Ye Y, Tang Y, Almoiliqy M, Adlat S, Wei J, Zhang Y, Li X. 2020; Indian Hedgehog regulates senescence in bone marrow-derived mesenchymal stem cell through modulation of ROS/mTOR/4EBP1, p70S6K1/2 pathway. Aging (Albany NY). 12:5693–5715. DOI: 10.18632/aging.102958. PMID: 32235006. PMCID: PMC7185126.
Article23. Atashi F, Modarressi A, Pepper MS. 2015; The role of reactive oxygen species in mesenchymal stem cell adipogenic and osteogenic differentiation: a review. Stem Cells Dev. 24:1150–1163. DOI: 10.1089/scd.2014.0484. PMID: 25603196. PMCID: PMC4424969.
Article24. Liu PC, Liu K, Liu JF, Xia K, Chen LY, Wu X. 2016; Transfection of the IHH gene into rabbit BMSCs in a simulated microgravity environment promotes chondrogenic differentiation and inhibits cartilage aging. Oncotarget. 7:62873–62885. DOI: 10.18632/oncotarget.11871. PMID: 27802423. PMCID: PMC5325333.
Article25. Al-Azab M, Wei J, Ouyang X, Elkhider A, Walana W, Sun X, Tang Y, Wang B, Li X. 2018; TL1A mediates fibroblast-like synoviocytes migration and Indian Hedgehog signaling pathway via TNFR2 in patients with rheumatoid arthritis. Eur Cytokine Netw. 29:27–35. DOI: 10.1684/ecn.2018.0405. PMID: 29748156.
Article26. Al-Azab M, Qaed E, Ouyang X, Elkhider A, Walana W, Li H, Li W, Tang Y, Adlat S, Wei J, Wang B, Li X. 2020; TL1A/TNFR2-mediated mitochondrial dysfunction of fibroblast-like synoviocytes increases inflammatory response in patients with rheumatoid arthritis via reactive oxygen species generation. FEBS J. 287:3088–3104. DOI: 10.1111/febs.15181. PMID: 31953914.
Article27. Redondo-Castro E, Cunningham C, Miller J, Martuscelli L, Aoulad-Ali S, Rothwell NJ, Kielty CM, Allan SM, Pinteaux E. 2017; Interleukin-1 primes human mesenchymal stem cells towards an anti-inflammatory and pro-trophic phenotype in vitro. Stem Cell Res Ther. 8:79. DOI: 10.1186/s13287-017-0531-4. PMID: 28412968. PMCID: PMC5393041.
Article28. Philipp D, Suhr L, Wahlers T, Choi YH, Paunel-Görgülü A. 2018; Preconditioning of bone marrow-derived mesenchymal stem cells highly strengthens their potential to promote IL-6-dependent M2b polarization. Stem Cell Res Ther. 9:286. DOI: 10.1186/s13287-018-1039-2. PMID: 30359316. PMCID: PMC6202843.
Article29. Ling L, Feng X, Wei T, Wang Y, Wang Y, Wang Z, Tang D, Luo Y, Xiong Z. 2019; Human amnion-derived mesenchymal stem cell (hAD-MSC) transplantation improves ovarian function in rats with premature ovarian insufficiency (POI) at least partly through a paracrine mechanism. Stem Cell Res Ther. 10:46. DOI: 10.1186/s13287-019-1136-x. PMID: 30683144. PMCID: PMC6347748.
Article30. Ma H, Zhang S, Xu Y, Zhang R, Zhang X. 2017; Analysis of differentially expressed microRNA of TNF-α-stimulated mesenchymal stem cells and exosomes from their culture supernatant. Arch Med Sci. 14:1102–1111. DOI: 10.5114/aoms.2017.70878. PMID: 30154894. PMCID: PMC6111343.
Article31. Lin T, Pajarinen J, Nabeshima A, Lu L, Nathan K, Jämsen E, Yao Z, Goodman SB. 2017; Preconditioning of murine mesenchymal stem cells synergistically enhanced immunomodu-lation and osteogenesis. Stem Cell Res Ther. 8:277. DOI: 10.1186/s13287-017-0730-z. PMID: 29212557. PMCID: PMC5719931.
Article32. Shen L, Zhang S, Zhang X, Zhang Y, Xie L, Jiang Y, Ma Y, Li G. 2016; Enhancing the ability of autophagy and proliferation of bone marrow mesenchymal stem cells by interleukin-8 through Akt-STAT3 pathway in hypoxic environment. Sheng Wu Gong Cheng Xue Bao. 32:1422–1432. Chinese. DOI: 10.13345/j.cjb.160035. PMID: 29027451.33. Yang A, Lu Y, Xing J, Li Z, Yin X, Dou C, Dong S, Luo F, Xie Z, Hou T, Xu J. 2018; IL-8 Enhances Therapeutic Effects of BMSCs on Bone Regeneration via CXCR2-Mediated PI3k/Akt Signaling Pathway. Cell Physiol Biochem. 48:361–370. DOI: 10.1159/000491742. PMID: 30016780.
Article34. Barhanpurkar-Naik A, Mhaske ST, Pote ST, Singh K, Wani MR. 2017; Interleukin-3 enhances the migration of human mesenchymal stem cells by regulating expression of CXCR4. Stem Cell Res Ther. 8:168. DOI: 10.1186/s13287-017-0618-y. PMID: 28705238. PMCID: PMC5512829.
Article35. Kapranov NM, Davydova YO, Galtseva IV, Petinati NA, Drize NI, Kuzmina LA, Parovichnikova EN, Savchenko VG. 2017; Effect of priming of multipotent mesenchymal stromal cells with interferon γ on their immunomodulating pro-perties. Biochemistry (Mosc). 82:1158–1168. DOI: 10.1134/S000629791710008X. PMID: 29037136.
Article36. Liang C, Jiang E, Yao J, Wang M, Chen S, Zhou Z, Zhai W, Ma Q, Feng S, Han M. 2018; Interferon-γ mediates the immunosuppression of bone marrow mesenchymal stem cells on T-lymphocytes in vitro. Hematology. 23:44–49. DOI: 10.1080/10245332.2017.1333245. PMID: 28581352.
Article37. Ho L, Alman B. 2010; Protecting the hedgerow: p53 and hedgehog pathway interactions. Cell Cycle. 9:506–511. DOI: 10.4161/cc.9.3.10552. PMID: 20081367.
Article38. Steinert AF, Weissenberger M, Kunz M, Gilbert F, Ghivizzani SC, Göbel S, Jakob F, Nöth U, Rudert M. 2012; Indian hedgehog gene transfer is a chondrogenic inducer of human mesenchymal stem cells. Arthritis Res Ther. 14:R168. DOI: 10.1186/ar3921. PMID: 22817660. PMCID: PMC3580562.
Article39. Lin T, Kohno Y, Huang JF, Romero-Lopez M, Pajarinen J, Maruyama M, Nathan K, Yao Z, Goodman SB. 2018; NFκB sensing IL-4 secreting mesenchymal stem cells mitigate the proinflammatory response of macrophages exposed to polyethylene wear particles. J Biomed Mater Res A. 106:2744–2752. DOI: 10.1002/jbm.a.36504. PMID: 30084534. PMCID: PMC6207939.
Article40. Tan CQ, Gao X, Guo L, Huang H. 2014; Exogenous IL-4-expressing bone marrow mesenchymal stem cells for the treatment of autoimmune sensorineural hearing loss in a guinea pig model. Biomed Res Int. 2014:856019. DOI: 10.1155/2014/856019. PMID: 24864261. PMCID: PMC4016942.41. Habib R, Haneef K, Naeem N, Khan I, Jamall S, Salim A. Atta-Ur-Rahman. 2015; Hypoxic stress and IL-7 gene overexpression enhance the fusion potential of rat bone marrow mesenchymal stem cells with bovine renal epithelial cells. Mol Cell Biochem. 403:125–137. DOI: 10.1007/s11010-015-2343-0. PMID: 25666089.
Article42. Ma T, Wang X, Jiao Y, Wang H, Qi Y, Gong H, Zhang L, Jiang D. 2018; Interleukin 17 (IL-17)-induced mesenchymal stem cells prolong the survival of allogeneic skin grafts. Ann Transplant. 23:615–621. DOI: 10.12659/AOT.909381. PMID: 30166501. PMCID: PMC6248056.
Article43. Sivanathan KN, Coates PT. 2018; IL-17A-induced mesenchymal stem cells have promising therapeutic value for clinical translation. Kidney Int. 93:771–773. DOI: 10.1016/j.kint.2017.12.010. PMID: 29571447.
Article44. Bie Q, Zhang B, Sun C, Ji X, Barnie PA, Qi C, Peng J, Zhang D, Zheng D, Su Z, Wang S, Xu H. 2017; IL-17B activated mesenchymal stem cells enhance proliferation and migration of gastric cancer cells. Oncotarget. 8:18914–18923. DOI: 10.18632/oncotarget.14835. PMID: 28145881. PMCID: PMC5386657.
Article45. El-Zayadi AA, Jones EA, Churchman SM, Baboolal TG, Cuthbert RJ, El-Jawhari JJ, Badawy AM, Alase AA, El-Sherbiny YM, McGonagle D. 2017; Interleukin-22 drives the proliferation, migration and osteogenic differentiation of mesenchymal stem cells: a novel cytokine that could contribute to new bone formation in spondyloarthropathies. Rheumatology (Oxford). 56:488–493. DOI: 10.1093/rheumatology/kew384. PMID: 27940584.
Article46. Rostami M, Haidari K, Shahbazi M. 2018; The human IL-23 decoy receptor inhibits T-cells producing IL-17 by genetically engineered mesenchymal stem cells. Int J Cell Biol. 2018:8213912. DOI: 10.1155/2018/8213912. PMID: 30662466. PMCID: PMC6313978.
Article47. Sriramulu S, Banerjee A, Di Liddo R, Jothimani G, Gopinath M, Murugesan R, Marotta F, Pathak S. 2018; Concise review on clinical applications of conditioned medium derived from human umbilical cord-mesenchymal stem cells (UC-MSCs). Int J Hematol Oncol Stem Cell Res. 12:230–234. PMID: 30595826. PMCID: PMC6305261.48. Mateos J, De la Fuente A, Lesende-Rodriguez I, Fernández-Pernas P, Arufe MC, Blanco FJ. 2013; Lamin A deregulation in human mesenchymal stem cells promotes an impairment in their chondrogenic potential and imbalance in their response to oxidative stress. Stem Cell Res. 11:1137–1148. DOI: 10.1016/j.scr.2013.07.004. PMID: 23994728.
Article49. Simic P, Zainabadi K, Bell E, Sykes DB, Saez B, Lotinun S, Baron R, Scadden D, Schipani E, Guarente L. 2013; SIRT1 regulates differentiation of mesenchymal stem cells by deacetylating β-catenin. EMBO Mol Med. 5:430–440. DOI: 10.1002/emmm.201201606. PMID: 23364955. PMCID: PMC3598082.
Article50. Xiao L, Sobue T, Esliger A, Kronenberg MS, Coffin JD, Doetschman T, Hurley MM. 2010; Disruption of the Fgf2 gene activates the adipogenic and suppresses the osteogenic program in mesenchymal marrow stromal stem cells. Bone. 47:360–370. DOI: 10.1016/j.bone.2010.05.021. PMID: 20510392. PMCID: PMC2947437.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Safety and Efficacy of Autologous Bone Marrow-Derived Mesenchymal Stem Cell Transplantation in Sensorineural Hearing Loss Patients
- Bone Marrow-Derived Mesenchymal Stem Cells for Regenerative Medicine
- Concise Review: Differentiation of Human Adult Stem Cells Into Hepatocyte-like Cells In vitro
- Neural Differentiation of Bone Marrow-Derived Mesenchymal Stem Cells: Applicability for Inner Ear Therapy
- Effect of the hedgehog signaling pathway on hair formation-related cells