Preparing for the Coronavirus Disease (COVID-19) Vaccination: Evidence, Plans, and Implications
- Affiliations
-
- 1Department of Preventive Medicine, Gachon University College of Medicine, Incheon, Korea
- 2Artificial Intelligence and Big-Data Convergence Center, Gachon University Gil Medical Center, Gachon University College of Medicine, Incheon, Korea
- KMID: 2513076
- DOI: http://doi.org/10.3346/jkms.2021.36.e59
Abstract
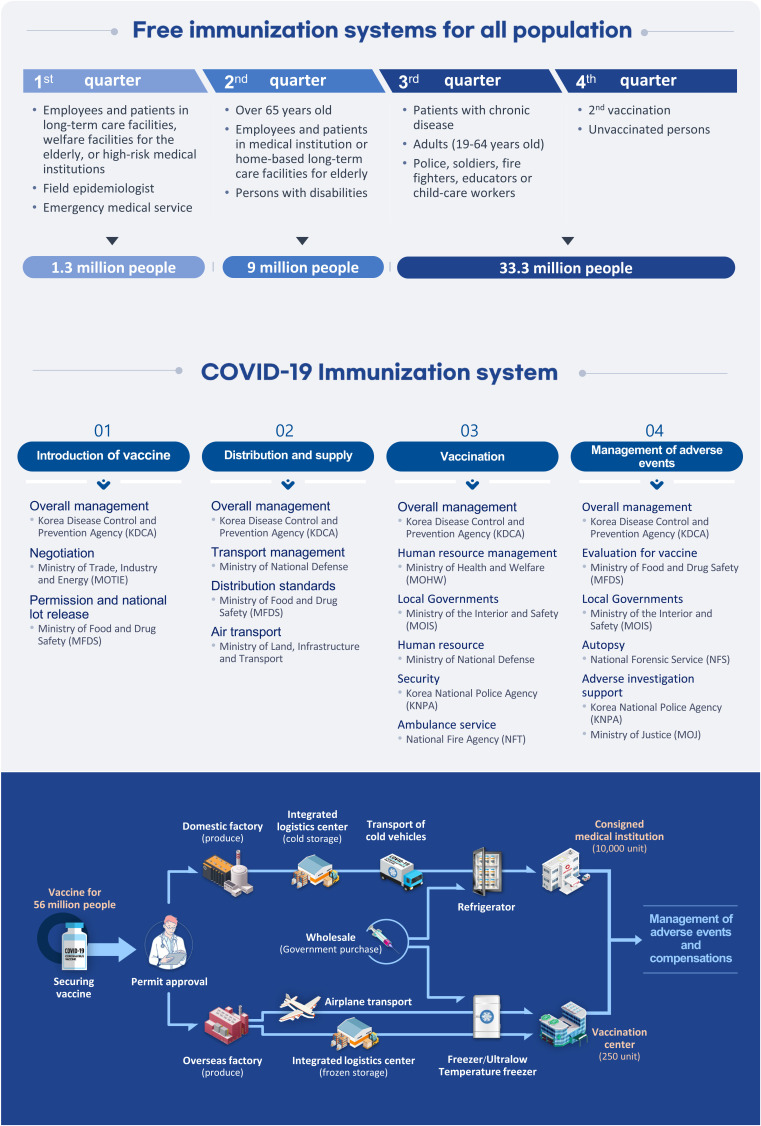
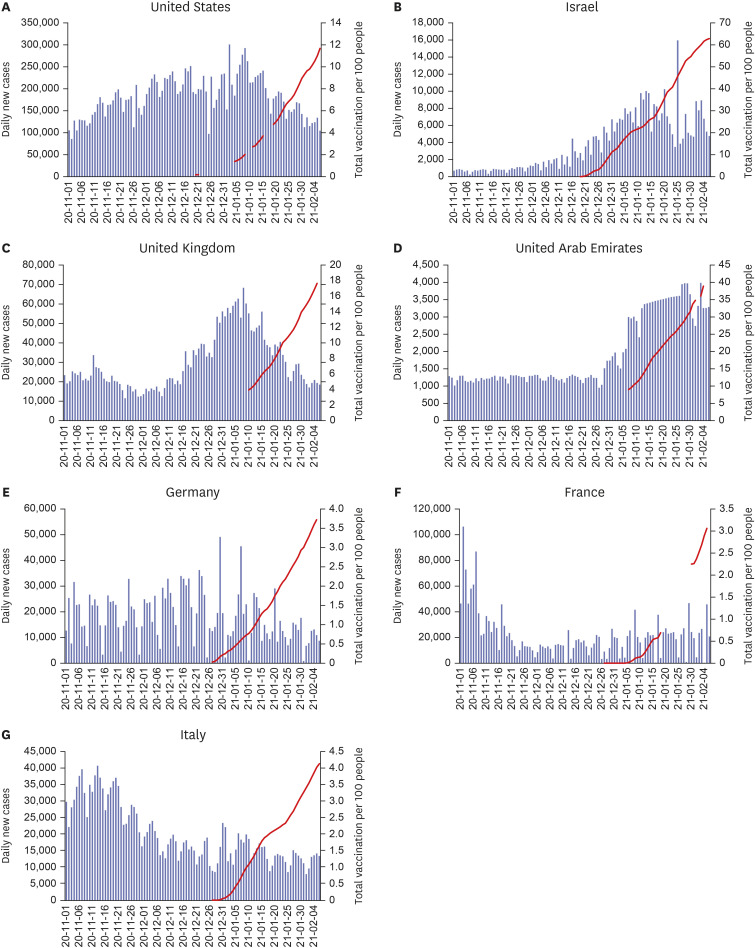
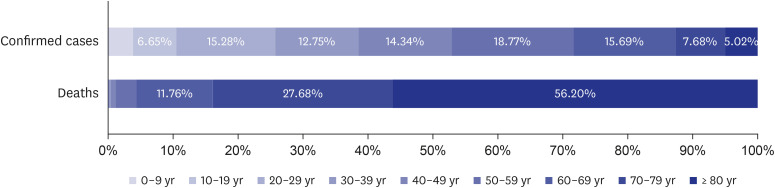
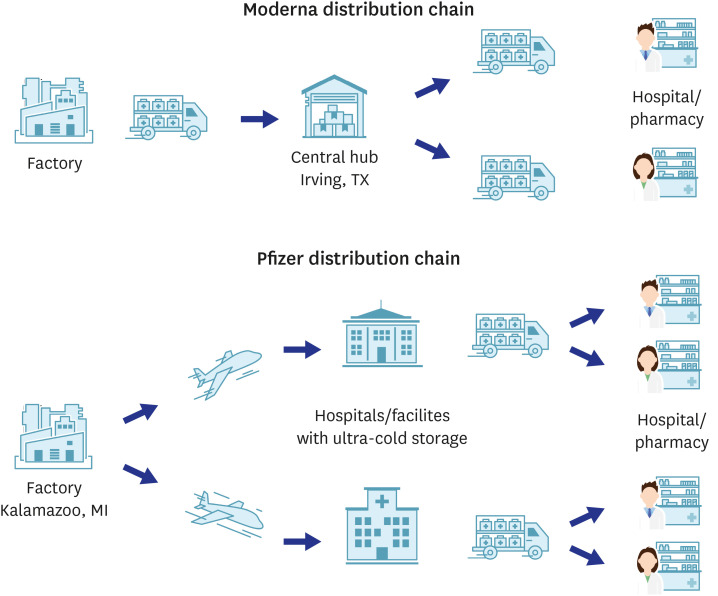
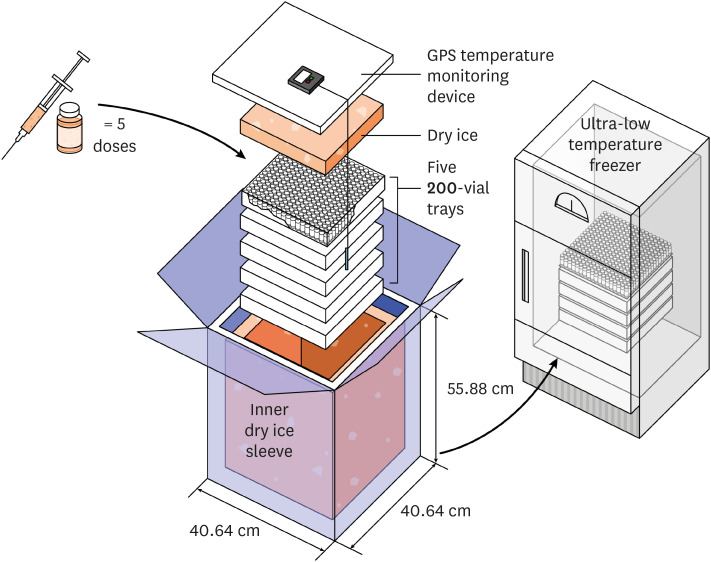
- The formation of herd immunity through vaccination is a key point in overcoming the coronavirus disease 2019 (COVID-19) pandemic. To acquire herd immunity, a high vaccination rate is required, which is necessary to instill confidence in the public regarding the effectiveness and safety of the vaccine. In the real-world setting, thorough preparation of components, such as priority setting, vaccine delivery, logistics, and side-effect monitoring is necessary to overcome vaccine hesitancy. Each country prioritizes vaccination since healthcare workers, nursing facility residents, and the elderly population, and similar trends are found between countries. Vaccination is performed at large centers and medical institutions operated by the country, and variations are dependent on the environment of each country. The transport of mRNA vaccines is a challenging task, and to this end, each government is striving for safe distribution. In addition, each authority operates a surveillance system to monitor the safety of vaccines, and Korea needs to produce evidence for monitoring effects and side effects with expertise. Even after the acquisition of herd immunity, COVID-19 is highly likely to remain an endemic infectious disease, and a higher immunity level may be required because of variants of the virus. If the spread of variants of concern continues, a booster vaccination may be required. Therefore, non-pharmaceutical interventions, such as social distancing, wearing a mask, and epidemiological investigation should be maintained.
Figure
Cited by 6 articles
-
Machine Learning Approach for Active Vaccine Safety Monitoring
Yujeong Kim, Jong-Hwan Jang, Namgi Park, Na-Young Jeong, Eunsun Lim, Soyun Kim, Nam-Kyong Choi, Dukyong Yoon
J Korean Med Sci. 2021;36(31):e198. doi: 10.3346/jkms.2021.36.e198.Why Fast COVID-19 Vaccination Needed for People with Disabilities and Autistics in Korea?
Wn-Ho Yoon
J Korean Med Sci. 2021;36(37):e267. doi: 10.3346/jkms.2021.36.e267.National Academy of Medicine of Korea (NAMOK) Key Statements on COVID-19
Hyoung-Shik Shin, Hyesook Park, Jun Soo Kwon, Hyun Namgoong, Seong-Jun Kim, June Myung Kim, Kyong Ran Peck, Kyungwon Lee, Jong-koo Lee, JinHan Lee, Hee Chul Han, SungJin Hong, Byung-Joo Park, Tae Hwan Lim, Eung Soo Hwang, Jun Hee Woo,
J Korean Med Sci. 2021;36(41):e287. doi: 10.3346/jkms.2021.36.e287.Comparison of Antibody Response Elicited by ChAdOx1 and BNT162b2 COVID-19 Vaccine
Yu Min Kang, Dohsik Minn, Jaegyun Lim, Ki-Deok Lee, Dong Ho Jo, Kang-Won Choe, Moon Jung Kim, Jong Min Kim, Kwang Nam Kim
J Korean Med Sci. 2021;36(46):e311. doi: 10.3346/jkms.2021.36.e311.Analyses of Confirmed COVID-19 Cases Among Korean Military Personnel After Mass Vaccination
Dong Hoon Shin, Hong Sang Oh, Haebong Jang, Sangho Lee, Byung Seop Choi, Donghoon Kim
J Korean Med Sci. 2022;37(3):e23. doi: 10.3346/jkms.2022.37.e23.Effective Vaccination and Education Strategies for Emerging Infectious Diseases Such as COVID-19
Seong-Heon Wie, Jaehun Jung, Woo Joo Kim
J Korean Med Sci. 2023;38(44):e371. doi: 10.3346/jkms.2023.38.e371.
Reference
-
1. McLellan A, Godlee F. Covid 19: Christmas relaxation will overwhelm services. BMJ. 2020; 371:m4847. PMID: 33323367.
Article2. Park Y, Huh IS, Lee J, Kang CR, Cho SI, Ham HJ, et al. Application of testing-tracing-treatment strategy in response to the COVID-19 outbreak in Seoul, Korea. J Korean Med Sci. 2020; 35(45):e396. PMID: 33230987.
Article3. Mummert A, Weiss H, Long LP, Amigó JM, Wan XF. A perspective on multiple waves of influenza pandemics. PLoS One. 2013; 8(4):e60343. PMID: 23637746.
Article4. Korea Centers for Disease Control & Prevention. Free Vaccination for All Citizens of COVID-19 to Return to Daily Life. Cheongju: Korea Centers for Disease Control & Prevention;2021.5. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020; 383(27):2603–2615. PMID: 33301246.
Article6. Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2020; 384(5):403–416. PMID: 33378609.
Article7. Johnson & Johnson. Johnson & Johnson announces single-shot Janssen COVID-19 vaccine candidate met primary endpoints in interim analysis of its Phase 3 ENSEMBLE Trial. Cited Feb 1, 2021. Available from: https://www.jnj.com/johnson-johnson-announces-single-shot-janssen-covid-19-vaccine-candidate-met-primary-endpoints-in-interim-analysis-of-its-phase-3-ensemble-trial.8. Novavax. Novavax COVID-19 Vaccine Demonstrates 89.3% Efficacy in UK Phase 3 Trial. Published January 28, 2021. Accessed Jan 31, 2021. Available from: https://ir.novavax.com/news-releases/news-release-details/novavax-covid-19-vaccine-demonstrates-893-efficacy-uk-phase-3.9. Muik A, Wallisch AK, Sänger B, Swanson KA, Mühl J, Chen W, et al. Neutralization of SARS-CoV-2 lineage B.1.1.7 pseudovirus by BNT162b2 vaccine-elicited human sera. Science.
Article10. Moderna. Moderna COVID-19 vaccine retains neutralizing activity against emerging variants first identified in the U.K. and the Republic of South Africa. Updated 2021. Accessed January 25, 2021. https://investors.modernatx.com/news-releases/news-release-details/moderna-covid-19-vaccine-retains-neutralizing-activity-against.11. Knoll MD, Wonodi C. Oxford-AstraZeneca COVID-19 vaccine efficacy. Lancet. 2021; 397(10269):72–74. PMID: 33306990.
Article12. Voysey M, Costa Clemens SA, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. A. Single dose administration, and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine. Updated 2021. Accessed January 28, 2021. https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3777268.13. Emary KFW, Golubchik T, Aley PK, Ariani CV, Angus BJ, Bibi S, et al. Efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 VOC 202012/01 (B.1.1.7). Updated 2021. Accessed February 9, 2021. https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3779160.14. The Guardian. Study shows Oxford Covid vaccine has less protection against South African variant. Updated 2021. Accessed February 9, 2021. https://www.theguardian.com/society/2021/feb/07/covid-vaccine-booster-variants-emerge-minister.15. Our World in Data. Coronavirus (COVID-19) vaccinations. Updated 2021. Accessed February 1, 2021. https://ourworldindata.org/covid-vaccinations.16. The Times of Israel. Vaccine found 92% effective in Israel, in first controlled result outside trials. Updated 2021. Accessed January 28, 2021. https://www.timesofisrael.com/vaccine-found-92-effective-in-israel-in-first-controlled-result-outside-trials/.17. Statistics Korea. COVID-19. Updated 2021. Accessed February 1, 2021. https://kosis.kr/covid/covid_index.do.18. Kim T. Improving preparedness for and response to coronavirus Disease 19 (COVID-19) in long-term care hospitals in Korea. Infect Chemother. 2020; 52(2):133–141. PMID: 32406211.
Article19. Chung H, Kim EO, Kim SH, Jung J. Risk of COVID-19 transmission from infected outpatients to healthcare workers in an outpatient clinic. J Korean Med Sci. 2020; 35(50):e431. PMID: 33372425.
Article20. Park C, Hwang JM, Jo S, Bae SJ, Sakong J. COVID-19 outbreak and its association with healthcare workers' emotional stress: a cross-sectional study. J Korean Med Sci. 2020; 35(41):e372. PMID: 33107230.
Article21. World Health Organization. Who sage road map for prioritizing uses of COVID-19 vaccines in the context of limited supply. Updated 2020. Accessed January 28, 2021. https://www.who.int/docs/default-source/immunization/sage/covid/sage-prioritization-roadmap-covid19-vaccines.pdf?Status=Temp&sfvrsn=bf227443_2.22. Dooling K, Marin M, Wallace M, McClung N, Chamberland M, Lee GM, et al. The advisory committee on immunization practices' updated interim recommendation for allocation of COVID-19 Vaccine - United States, December 2020. MMWR Morb Mortal Wkly Rep. 2021; 69(5152):1657–1660. PMID: 33382671.
Article23. Joint Committee on Vaccination and Immunisation. Advice on priority groups for COVID-19 vaccination. Updated 2020. Accessed January 28, 2021. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/950113/jcvi-advice-on-priority-groups-for-covid-19-vaccination-30-dec-2020-revised.pdf.24. Vygen-Bonnet S, Koch J, Bogdan C, Harder T, Heininger U, Kling K, et al. Beschluss der STIKO zur 1. Aktualisierung der COVID-19-Impfempfehlung und die dazugehörige wissenschaftliche Begründung. Epidemiol Bull. 2021; 2:64–132.25. Australian Technical Advisory Group on Immunisation. Preliminary advice on general principles to guide the prioritisation of target populations in a COVID-19 vaccination program in Australia. Updated 2020. Accessed January 28, 2021. https://www.health.gov.au/sites/default/files/documents/2020/11/atagi-preliminary-advice-on-general-principles-to-guide-the-prioritisation-of-target-populations-in-a-covid-19-vaccination-program-in-australia_0.pdf.26. National Advisory Committee on Immunization. Guidance on the prioritization of initial doses of COVID-19 vaccine(s). Updated 2020. Accessed January 28, 2021. https://www.canada.ca/en/public-health/services/immunization/national-advisory-committee-on-immunization-naci/guidance-prioritization-initial-doses-covid-19-vaccines.html.27. French National Authority for Health. COVID-19 vaccines: what prioritization strategy at the start of the campaign? Updated 2020. Accessed January 28, 2021. https://www.has-sante.fr/jcms/p_3221237/en/vaccins-covid-19-quelle-strategie-de-priorisation-a-l-initiation-de-la-campagne.28. Folkhälsomyndigheten. Nationell plan för vaccinationmot COVID-19. Updated 2020. Accessed January 28, 2021. https://www.folkhalsomyndigheten.se/contentassets/f8703f0a29cc408fb788b60f87289e5b/nationell-plan-vaccination-covid-19.pdf.29. The Local. COVID-19: what's Sweden's priority list for the vaccine? Updated 2020. Accessed January 28, 2021. https://www.thelocal.se/20201209/covid-19-whats-swedens-priority-list-for-the-vaccine.30. Japantimes. Japan's COVID-19 vaccine plan prioritizes health care workers and older residents. Updated 2020. Accessed January 28, 2021. https://www.japantimes.co.jp/news/2020/12/25/national/japan-vaccine-older-people/.31. Washingtonpost. Outpacing Trump's operation warp speed, China to give coronavirus vaccine to 50 million in a month. Updated 2021. Accessed January 28, 2021. https://www.washingtonpost.com/world/asia_pacific/coronavirus-vaccine-china-lunar-new-year/2021/01/04/d9401354-4e34-11eb-a1f5-fdaf28cfca90_story.html.32. Ministry of Health. Everything you need to know COVID-19 VACCINE. Updated 2020. Accessed January 28, 2021. https://www.moh.gov.sa/awarenessplateform/VariousTopics/Documents/COVID-19Vaccine-English.pdf.33. Ministry of Health. News. MOH announces priority groups for COVID-19 vaccination;Updated 2020. Accessed January 28, 2021. https://www.moh.gov.sa/en/Ministry/MediaCenter/News/Pages/News-2020-12-15-008.aspx.34. Ministry of Health, Division of Epidemiology. COVID-19 vaccine priorities. Updated 2020. Accessed January 28, 2021. https://www.gov.il/BlobFolder/news/16122020-01/he/NEWS_Corona_corona-vaccine-priorities.pdf.35. Ministry of Health. COVID-19 vaccination information. Updated 2021. Accessed January 28, 2021. https://govextra.gov.il/ministry-of-health/covid19-vaccine/en-covid19-vaccination-information/.36. Department of Health & Human Services. Fact sheet: explaining operation warp speed. Updated 2021. Accessed January 28, 2021. https://www.hhs.gov/coronavirus/explaining-operation-warp-speed/index.html.37. Department of Health & Social Care. UK COVID-19 vaccines delivery plan. Updated 2021. Accessed January 28, 2021. https://www.gov.uk/government/publications/uk-covid-19-vaccines-delivery-plan/uk-covid-19-vaccines-delivery-plan.38. Robert Koch Institute. COVID-19 and vaccination: Answers to frequently asked questions (FAQ). Updated 2021. Accessed January 28, 2021. Available from: https://www.rki.de/SharedDocs/FAQ/COVID-Impfen/gesamt.html.39. Australian Government. Australia's COVID-19 vaccine national roll-out strategy. Updated 2021. Accessed January 28, 2021. https://www.health.gov.au/resources/publications/australias-covid-19-vaccine-national-roll-out-strategy.40. British of Columbia. COVID-19 immunization plan. Updated 2021. Accessed January 28, 2021. https://www2.gov.bc.ca/gov/content/safety/emergency-preparedness-response-recovery/covid-19-provincial-support/vaccines.41. Ontario. Ontario expands COVID-19 vaccine locations. Updated 2020. Accessed January 28, 2021. https://news.ontario.ca/en/release/59753/ontario-expands-covid-19-vaccine-locations.42. Ministère des solidarités, de la santé. The vaccine strategy. Updated 2020. Accessed January 28, 2021. https://solidarites-sante.gouv.fr/grands-dossiers/la-vaccination-contre-la-covid-19/article/la-strategie-vaccinale.43. Riyadh- Asharq Al-Awsat. High registration turnout to receive COVID-19 vaccine in Saudi Arabia. Updated 2021. Accessed January 28, 2021. https://english.aawsat.com/home/article/2751411/high-registration-turnout-receive-covid-19-vaccine-saudi-arabia.44. Ministry of Health. The Minister of Health held tonight (Monday) a status assessment at the vaccine control center. Updated 2020. Accessed January 28, 2021. https://www.gov.il/en/departments/news/21122020-05.45. The Wall Street Journal. U.S. starts delivery of Moderna's COVID-19 vaccine. Updated 2020. Accessed January 28, 2021. https://www.wsj.com/articles/u-s-starts-rollout-of-modernas-covid-19-vaccine-11608460200.46. CNBC. Pfizer CNBC's COVID vaccine is now shipping. Here's how the U.S. plans to deliver it. Updated 2020. Accessed January 28, 2021. https://www.cnbc.com/2020/12/12/how-fedex-ups-plan-to-distribute-fda-approved-covid-vaccine-when-will-you-get-the-coronavirus-vaccine.html.47. Department of Health. Contracts signed for roll out of COVID-19 vaccine. Updated 2020. Accessed January 28, 2021. https://www.health.gov.au/ministers/the-hon-greg-hunt-mp/media/contracts-signed-for-rollout-of-covid-19-vaccine.48. Government of Canada. Canada's COVID-19 immunization plan: saving lives and livelihoods. Updated 2020. Accessed January 28, 2021. https://www.canada.ca/en/public-health/services/diseases/2019-novel-coronavirus-infection/canadas-reponse/canadas-covid-19-immunization-plan.html.49. CDC COVID-19 Response Team. Food and Drug Administration. Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine—United States, December 14–23, 2020. MMWR Morb Mortal Wkly Rep. 2021; 70(2):46–51. PMID: 33444297.50. Cabanillas B, Akdis C, Novak N. Allergic reactions to the first COVID-19 vaccine: a potential role of Polyethylene glycol? Allergy.
Article51. CDC. Interim clinical considerations for use of mRNA COVID-19 vaccines currently authorized in the United States. Updated 2021. Accessed January 28, 2021. https://www.cdc.gov/vaccines/covid-19/info-by-product/clinical-considerations.html.52. Culbreth MJ, Biryukov SS, Shoe JL, Dankmeyer JL, Hunter M, Klimko CP, et al. The use of analgesics during vaccination with a live attenuated Yersinia pestis vaccine alters the resulting immune response in mice. Vaccines (Basel). 2019; 7(4):205.
Article53. Saleh E, Moody MA, Walter EB. Effect of antipyretic analgesics on immune responses to vaccination. Hum Vaccin Immunother. 2016; 12(9):2391–2402. PMID: 27246296.
Article54. Iwasaki A. What reinfections mean for COVID-19. Lancet Infect Dis. 2021; 21(1):3–5. PMID: 33058796.
Article55. Jung J. Epidemiologic evaluation and risk communication regarding the recent reports of sudden death after influenza vaccination in the COVID-19 pandemic. J Korean Med Sci. 2020; 35(41):e378. PMID: 33107233.
Article56. Lee YR, Lee JY, Park IH, Kim M, Jhon M, Kim JW, et al. The relationships among media usage regarding COVID-19, knowledge about infection, and anxiety: Structural model analysis. J Korean Med Sci. 2020; 35(48):e426. PMID: 33316862.
Article57. CDC. V-safe after vaccination health checker. Updated 2021. Accessed February 1, 2021. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/vsafe.html.58. CDC. Vaccine safety Datalink (VSD). Updated 2020. Accessed February 1, 2021. https://www.cdc.gov/vaccinesafety/ensuringsafety/monitoring/vsd/index.html.59. CDC. Ensuring the safety of COVID-19 vaccines in the United States. Updated 2021. Accessed February 1, 2021. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety.html.60. Parliament UK. Monitoring COVID-19 vaccine safety in national immunisation programmes. Updated 2021. Accessed February 1, 2021. https://post.parliament.uk/monitoring-covid-19-vaccine-safety-in-national-immunisation-programmes/.61. Paul-Ehrlich-Institut. Coronavirus and COVID-19. Updated 2021. Accessed February 1, 2021. https://www.pei.de/EN/newsroom/dossier/coronavirus/coronavirus-content.html;jsessionid=19526C06D00953CBA2E1084998E29C87.intranet232?nn=164146&cms_pos=5.62. National Centre for Immunisation Research and Surveillance. Vaccine safety. Updated 2020. Accessed February 1, 2021. https://www.ncirs.org.au/health-professionals/vaccine-safety.63. Australian Government. COVID-19 vaccine: information for consumers and health professionals. Updated 2021. Accessed February 1, 2021. https://www.tga.gov.au/covid-19-vaccine-information-consumers-and-health-professionals#report-side-effect.64. Government of Canada. Canadian Adverse Events Following Immunization Surveillance System (CAEFISS). Updated 2019. Accessed February 1, 2021. https://www.canada.ca/en/public-health/services/immunization/canadian-adverse-events-following-immunization-surveillance-system-caefiss.html.65. The Connexion. COVID-19 vaccine in France: side-effect monitoring increased. Updated 2021. Accessed February 1, 2021. https://www.connexionfrance.com/French-news/Covid-19-vaccine-in-France-Side-effect-monitoring-increased-as-vaccination-rolled-out.66. Swedish Medical Products Agency. Management of suspected adverse reaction reports. Updated 2020. Accessed February 1, 2021. https://www.lakemedelsverket.se/en/reporting-adverse-reactions-events-and-incidents/suspected-adverse-reactions-from-medicinal-products/medicinal-products-for-humans/management-of-suspected-adverse-reaction-reports.67. Ministry of Health. COVID-19 vaccine FAQs. Updated 2021. Accessed February 1, 2021. https://articlesen.covid19awareness.sa/COVID-19-Vaccine-FAQs.68. Jung J, Noh JY, Cheong HJ, Kim WJ, Song JY. Coronavirus disease 2019 outbreak at nightclubs and distribution centers after easing social distancing: vulnerable points of infection. J Korean Med Sci. 2020; 35(27):e247. PMID: 32657088.
Article69. Volz E, Mishra S, Chand M, Barrett JC, Johnson R, Geidelberg L, et al. Transmission of SARS-CoV-2 Lineage B.1.1.7 in England: insights from linking epidemiological and genetic data. Updated 2021. Accessed February 1, 2021. https://www.medrxiv.org/content/10.1101/2020.12.30.20249034v2.70. Naveca F, Nascimento V, Souza V, Corado A, Nascimento F, Silva G, et al. Phylogenetic relationship of SARS-CoV-2 sequences from Amazonas with emerging Brazilian variants harboring mutations E484K and N501Y in the Spike protein. Updated 2021. Accessed February 1, 2021. https://virological.org/t/phylogenetic-relationship-of-sars-cov-2-sequences-from-amazonas-with-emerging-brazilian-variants-harboring-mutations-e484k-and-n501y-in-the-spike-protein/585.71. Tanne JH. COVID-19: Moderna plans booster doses to counter variants. BMJ. 2021; 372(232):n232. PMID: 33500251.
Article72. Jung J, Jang H, Kim HK, Kim J, Kim A, Ko KP. The importance of mandatory COVID-19 diagnostic testing prior to release from quarantine. J Korean Med Sci. 2020; 35(34):e314. PMID: 32864911.
Article73. Park IN, Yum HK. Stepwise strategy of social distancing in Korea. J Korean Med Sci. 2020; 35(28):e264. PMID: 32686376.
Article74. Gov UK. Check if you can claim a grant through the self-employment income support scheme. Updated 2021. February 2, 2021. https://www.gov.uk/guidance/claim-a-grant-through-the-coronavirus-covid-19-self-employment-income-support-scheme#other-help-you-can-get/.75. Coronavirus.gov. How do I apply for small business or self-employment benefits? Updated 2020. February 2, 2021. https://faq.coronavirus.gov/how-to-apply-for-benefits/.76. de l'Économie M. des Finances et de la Relance. Coronavirus COVID-19: Soutien aux entreprises. Updated 2021. February 2, 2021. https://www.economie.gouv.fr/covid19-soutien-entreprises/fonds-de-solidarite-pour-les-tpe-independants-et-micro#/.77. General KP. Information. Updated 2021. Accessed February 2, 2021. https://home.kpmg/xx/en/home/insights/2020/04/japan-government-and-institution-measures-in-response-to-covid.html.78. Kyodo News. Japan's ruling bloc agrees on rent relief plan for virus-hit firms. Updated 2020. Accessed February 2, 2021. Available from: https://english.kyodonews.net/news/2020/05/cfd002fb6b3c-update1-japans-ruling-bloc-agrees-on-rent-relief-plan-for-virus-hit-firms.html.79. The Federal Government. Temporary support for small and medium businesses. Updated 2020. Accessed February 2, 2021. https://www.bundesregierung.de/breg-en/search/ueberbrueckungshilfe-1760136.80. Australian Government. Government response to coronavirus. Updated 2020. Accessed February 2, 2021. https://guides.dss.gov.au/guide-social-security-law/coronavirus.81. Australian Taxation Office. Instant asset write-off for eligible businesses. Updated 2020. Accessed February 2, 2021. https://www.ato.gov.au/Business/Depreciation-and-capital-expenses-and-allowances/Simpler-depreciation-for-small-business/Instant-asset-write-off/.82. Lexology. Taiwan: COVID-19 relief measures for employees. Updated 2020. Accessed February 2, 2021. https://www.lexology.com/library/detail.aspx?g=83f1285d-94f4-4b0e-8e48-fe36bf7017aa.83. Yoon Y, Kim KR, Park H, Kim S, Kim YJ. Stepwise school opening and an impact on the epidemiology of COVID-19 in the children. J Korean Med Sci. 2020; 35(46):e414. PMID: 33258334.
Article84. Kim EY, Ryu B, Kim EK, Park YJ, Choe YJ, Park HK, et al. Children with COVID-19 after reopening of schools, South Korea. Pediatr Infect Vaccine. 2020; 27(3):180–183.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Treatment for Immune Thrombocytopenia in Coronavirus Disease 2019 (COVID-19) Infection after COVID-19 Vaccination: A Case Report
- COVID-19 Vaccination and Herd Immunity
- A Case of Aphthous Stomatitis in a Healthy Adult Following COVID-19 Vaccination: Clinical Reasoning
- Early-Onset Myasthenia Gravis Following COVID-19 Vaccination
- Clinical implications of coronavirus disease 2019 in neonates