Differential Profile of Plasma Circular RNAs in Type 1 Diabetes Mellitus
- Affiliations
-
- 1Department of Endocrinology, The Second Hospital of Jilin University, Changchun, China.
- 2Department of Endocrinology, Sir Run Run Hospital, Nanjing Medical University, Nanjing, China.
- 3Department of Endocrinology, Children's Hospital of Nanjing Medical University, Nanjing, China.
- 4Division of Arthritis and Rheumatic Diseases, Oregon Health & Science University School of Medicine, Portland, OR, USA.
- 5Section of Rheumatology, VA Portland Health Care System, Portland, OR, USA.
- KMID: 2513049
- DOI: http://doi.org/10.4093/dmj.2019.0151
Abstract
Background No currently available biomarkers or treatment regimens fully meet therapeutic needs of type 1 diabetes mellitus (T1DM). Circular RNA (circRNA) is a recently identified class of stable noncoding RNA that have been documented as potential biomarkers for various diseases. Our objective was to identify and analyze plasma circRNAs altered in T1DM.
Methods We used microarray to screen differentially expressed plasma circRNAs in patients with new onset T1DM (
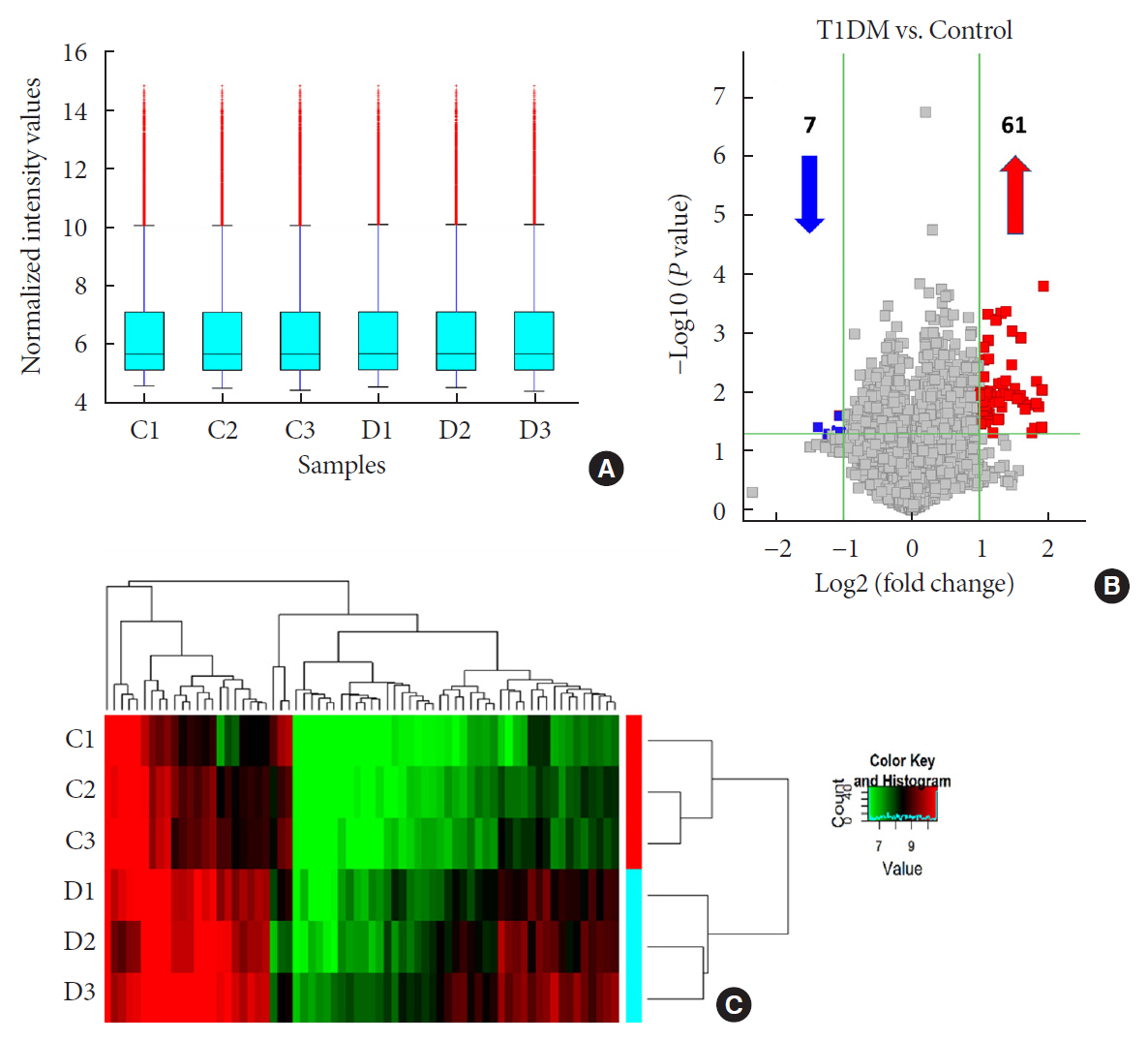
n =3) and age-/gender-matched healthy controls (n =3). Then, we selected six candidates with highest fold-change and validated them by quantitative real-time polymerase chain reaction in independent human cohort samples (n =12). Bioinformatic tools were adopted to predict putative microRNAs (miRNAs) sponged by these validated circRNAs and their downstream messenger RNAs (mRNAs). Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analyses were performed to gain further insights into T1DM pathogenesis.Results We identified 68 differentially expressed circRNAs, with 61 and seven being up- and downregulated respectively. Four of the six selected candidates were successfully validated. Curations of their predicted interacting miRNAs revealed critical roles in inflammation and pathogenesis of autoimmune disorders. Functional relations were visualized by a circRNA-miRNA-mRNA network. GO and KEGG analyses identified multiple inflammation-related processes that could be potentially associated with T1DM pathogenesis, including cytokine-cytokine receptor interaction, inflammatory mediator regulation of transient receptor potential channels and leukocyte activation involved in immune response.
Conclusion Our study report, for the first time, a profile of differentially expressed plasma circRNAs in new onset T1DM. Further
in silico annotations and bioinformatics analyses supported future application of circRNAs as novel biomarkers of T1DM.
Keyword
Figure
Reference
-
1. Chiang JL, Kirkman MS, Laffel LM, Peters AL. Type 1 Diabetes Sourcebook Authors. Type 1 diabetes through the life span: a position statement of the American Diabetes Association. Diabetes Care. 2014; 37:2034–2054.
Article2. Tao B, Pietropaolo M, Atkinson M, Schatz D, Taylor D. Estimating the cost of type 1 diabetes in the U.S.: a propensity score matching method. PLoS One. 2010; 5:e11501.
Article3. Bonifacio E. Predicting type 1 diabetes using biomarkers. Diabetes Care. 2015; 38:989–996.
Article4. Jin Y, She JX. Novel biomarkers in type 1 diabetes. Rev Diabet Stud. 2012; 9:224–235.
Article5. Chen LL. The biogenesis and emerging roles of circular RNAs. Nat Rev Mol Cell Biol. 2016; 17:205–211.
Article6. Salzman J. Circular RNA expression: its potential regulation and function. Trends Genet. 2016; 32:309–316.
Article7. You X, Vlatkovic I, Babic A, Will T, Epstein I, Tushev G, Akbalik G, Wang M, Glock C, Quedenau C, Wang X, Hou J, Liu H, Sun W, Sambandan S, Chen T, Schuman EM, Chen W. Neural circular RNAs are derived from synaptic genes and regulated by development and plasticity. Nat Neurosci. 2015; 18:603–610.
Article8. Lukiw WJ. Circular RNA (circRNA) in Alzheimer's disease (AD). Front Genet. 2013; 4:307.
Article9. Conn SJ, Pillman KA, Toubia J, Conn VM, Salmanidis M, Phillips CA, Roslan S, Schreiber AW, Gregory PA, Goodall GJ. The RNA binding protein quaking regulates formation of circRNAs. Cell. 2015; 160:1125–1134.
Article10. Burd CE, Jeck WR, Liu Y, Sanoff HK, Wang Z, Sharpless NE. Expression of linear and novel circular forms of an INK4/ARF-associated non-coding RNA correlates with atherosclerosis risk. PLoS Genet. 2010; 6:e1001233.
Article11. Fang Y, Wang X, Li W, Han J, Jin J, Su F, Zhang J, Huang W, Xiao F, Pan Q, Zou L. Screening of circular RNAs and validation of circANKRD36 associated with inflammation in patients with type 2 diabetes mellitus. Int J Mol Med. 2018; 42:1865–1874.
Article12. Zhao Z, Li X, Jian D, Hao P, Rao L, Li M. Hsa_circ_0054633 in peripheral blood can be used as a diagnostic biomarker of pre-diabetes and type 2 diabetes mellitus. Acta Diabetol. 2017; 54:237–245.
Article13. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2013; 36 Suppl 1:S67–S74.14. Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998; 15:539–553.
Article15. Rabinovitch A, Suarez-Pinzon WL. Role of cytokines in the pathogenesis of autoimmune diabetes mellitus. Rev Endocr Metab Disord. 2003; 4:291–299.16. Halling ML, Kjeldsen J, Knudsen T, Nielsen J, Hansen LK. Patients with inflammatory bowel disease have increased risk of autoimmune and inflammatory diseases. World J Gastroenterol. 2017; 23:6137–6146.
Article17. Liao KP, Gunnarsson M, Kallberg H, Ding B, Plenge RM, Padyukov L, Karlson EW, Klareskog L, Askling J, Alfredsson L. Specific association of type 1 diabetes mellitus with anti-cyclic citrullinated peptide-positive rheumatoid arthritis. Arthritis Rheum. 2009; 60:653–660.
Article18. Ludvigsson JF, Ludvigsson J, Ekbom A, Montgomery SM. Celiac disease and risk of subsequent type 1 diabetes: a general population cohort study of children and adolescents. Diabetes Care. 2006; 29:2483–2488.
Article19. Marrosu MG, Cocco E, Lai M, Spinicci G, Pischedda MP, Contu P. Patients with multiple sclerosis and risk of type 1 diabetes mellitus in Sardinia, Italy: a cohort study. Lancet. 2002; 359:1461–1465.
Article20. Fu Y, Yi Z, Li J, Li R. Deregulated microRNAs in CD4+ T cells from individuals with latent tuberculosis versus active tuberculosis. J Cell Mol Med. 2014; 18:503–513.21. Li R, Shen Q, Wu N, He M, Liu N, Huang J, Lu B, Yao Q, Yang Y, Hu R. MiR-145 improves macrophage-mediated inflammation through targeting Arf6. Endocrine. 2018; 60:73–82.
Article22. Balasa B, Van Gunst K, Sarvetnick N. The microbial product lipopolysaccharide confers diabetogenic potential on the T cell repertoire of BDC2.5/NOD mice: implications for the etiology of autoimmune diabetes. Clin Immunol. 2000; 95:93–98.
Article23. Luo Q, Zhang L, Li X, Fu B, Deng Z, Qing C, Su R, Xu J, Guo Y, Huang Z, Li J. Identification of circular RNAs hsa_circ_0044235 in peripheral blood as novel biomarkers for rheumatoid arthritis. Clin Exp Immunol. 2018; 194:118–124.
Article24. Dolati S, Ahmadi M, Aghebti-Maleki L, Nikmaram A, Marofi F, Rikhtegar R, Ayromlou H, Yousefi M. Nanocurcumin is a potential novel therapy for multiple sclerosis by influencing inflammatory mediators. Pharmacol Rep. 2018; 70:1158–1167.
Article25. Kawasaki E. Type 1 diabetes and autoimmunity. Clin Pediatr Endocrinol. 2014; 23:99–105.
Article26. Mansouri L, Lundwall K, Moshfegh A, Jacobson SH, Lundahl J, Spaak J. Vitamin D receptor activation reduces inflammatory cytokines and plasma MicroRNAs in moderate chronic kidney disease: a randomized trial. BMC Nephrol. 2017; 18:161.
Article27. Nakaoka H, Hirono K, Yamamoto S, Takasaki I, Takahashi K, Kinoshita K, Takasaki A, Nishida N, Okabe M, Ce W, Miyao N, Saito K, Ibuki K, Ozawa S, Adachi Y, Ichida F. MicroRNA-145-5p and microRNA-320a encapsulated in endothelial microparticles contribute to the progression of vasculitis in acute Kawasaki Disease. Sci Rep. 2018; 8:1016.
Article28. Eizirik DL, Darville MI. Beta-cell apoptosis and defense mechanisms: lessons from type 1 diabetes. Diabetes. 2001; 50 Suppl 1:S64–S69.
Article29. Jimenez JM, Boyall D, Brenchley G, Collier PN, Davis CJ, Fraysse D, Keily SB, Henderson J, Miller A, Pierard F, Settimo L, Twin HC, Bolton CM, Curnock AP, Chiu P, Tanner AJ, Young S. Design and optimization of selective protein kinase C θ (PKCθ) inhibitors for the treatment of autoimmune diseases. J Med Chem. 2013; 56:1799–1810.
Article30. Salzer E, Santos-Valente E, Keller B, Warnatz K, Boztug K. Protein kinase C δ: a gatekeeper of immune homeostasis. J Clin Immunol. 2016; 36:631–640.
Article31. Salzer E, Santos-Valente E, Klaver S, Ban SA, Emminger W, Prengemann NK, Garncarz W, Mullauer L, Kain R, Boztug H, Heitger A, Arbeiter K, Eitelberger F, Seidel MG, Holter W, Pollak A, Pickl WF, Forster-Waldl E, Boztug K. B-cell deficiency and severe autoimmunity caused by deficiency of protein kinase C δ. Blood. 2013; 121:3112–3116.
Article32. Hanes CM, D'Amico AE, Ueyama T, Wong AC, Zhang X, Hynes WF, Barroso MM, Cady NC, Trebak M, Saito N, Lennartz MR. Golgi-associated protein kinase C-ε is delivered to phagocytic cups: role of phosphatidylinositol 4-phosphate. J Immunol. 2017; 199:271–277.
Article33. Silva-Garcia O, Valdez-Alarcon JJ, Baizabal-Aguirre VM. The Wnt/β-catenin signaling pathway controls the inflammatory response in infections caused by pathogenic bacteria. Mediators Inflamm. 2014; 2014:310183.34. Suryawanshi A, Tadagavadi RK, Swafford D, Manicassamy S. Modulation of inflammatory responses by Wnt/β-catenin signaling in dendritic cells: a novel immunotherapy target for autoimmunity and cancer. Front Immunol. 2016; 7:460.
Article35. Nilius B, Owsianik G, Voets T, Peters JA. Transient receptor potential cation channels in disease. Physiol Rev. 2007; 87:165–217.
Article36. Tsuji F, Aono H. Role of transient receptor potential vanilloid 1 in inflammation and autoimmune diseases. Pharmaceuticals (Basel). 2012; 5:837–852.
Article37. Bluestone JA, Herold K, Eisenbarth G. Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature. 2010; 464:1293–1300.38. Insel RA, Dunne JL, Atkinson MA, Chiang JL, Dabelea D, Gottlieb PA, Greenbaum CJ, Herold KC, Krischer JP, Lernmark Å, Ratner RE, Rewers MJ, Schatz DA, Skyler JS, Sosenko JM, Ziegler AG. Staging presymptomatic type 1 diabetes: a scientific statement of JDRF, the Endocrine Society, and the American Diabetes Association. Diabetes Care. 2015; 38:1964–1974.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Role of Plasma Adiponectin and Polymorphism of Adiponectin Gene in the Development of Type 2 Diabetes Mellitus
- Effect of acipimox(olbetam)treatment on plasma lipids and glucose in hyperlipidemic patients with type 2 diabetes mellitus
- Plasma Paraoxonase Activities in Type 2 Diabetes Mellitus
- Differential Diagnosis of Diabetes Mellitus caused by Liver Cirrhosis and Other Type 2 Diabetes Mellitus
- Changes of plasma endothelin in non-insulin dependent diabetes mellitus