Time in Range from Continuous Glucose Monitoring: A Novel Metric for Glycemic Control
- Affiliations
-
- 1Division of Endocrinology and Metabolism, Department of Medicine, Yonsei University Wonju College of Medicine, Wonju, Korea
- 2Division of Endocrinology and Metabolism, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- KMID: 2513046
- DOI: http://doi.org/10.4093/dmj.2020.0257
Abstract
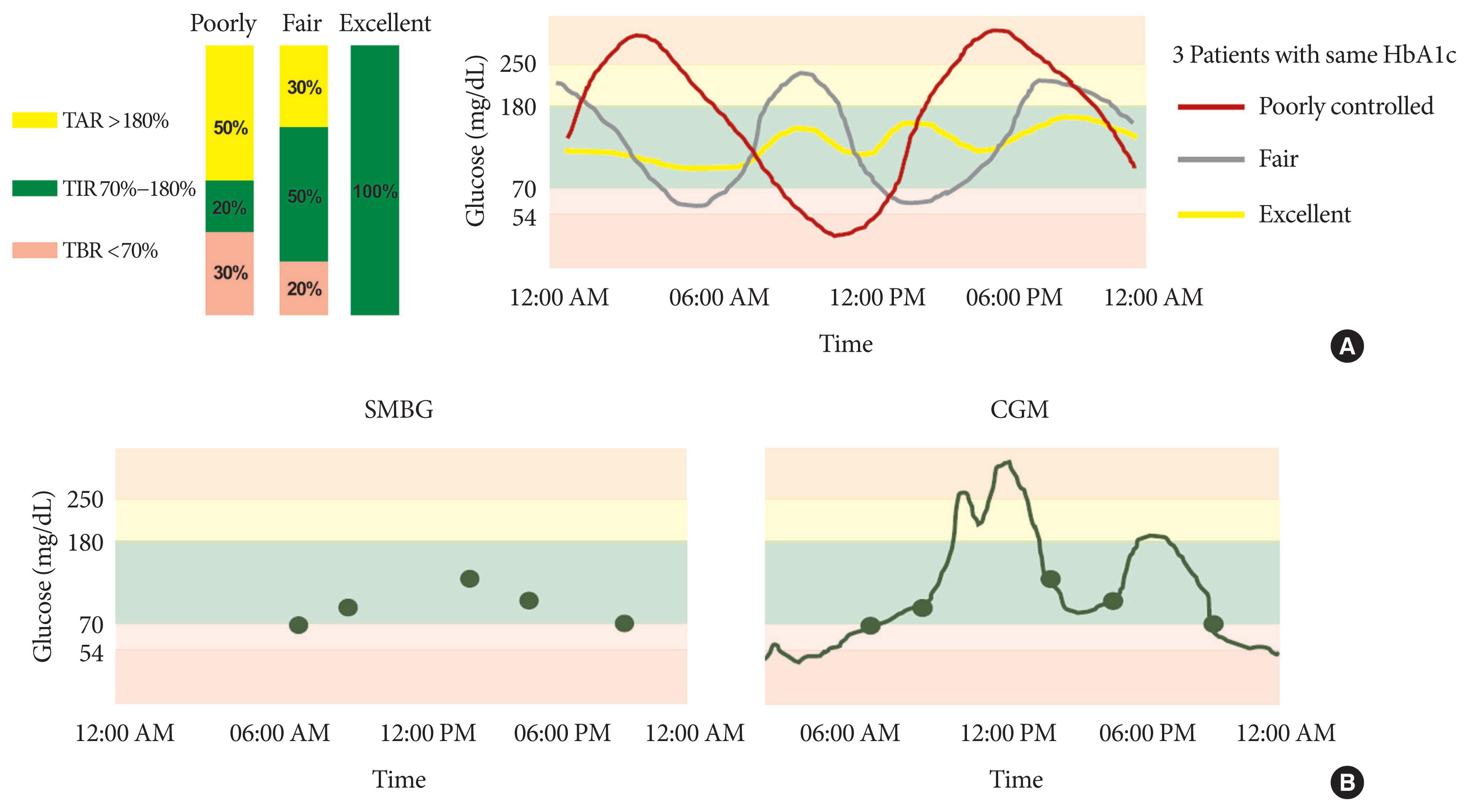
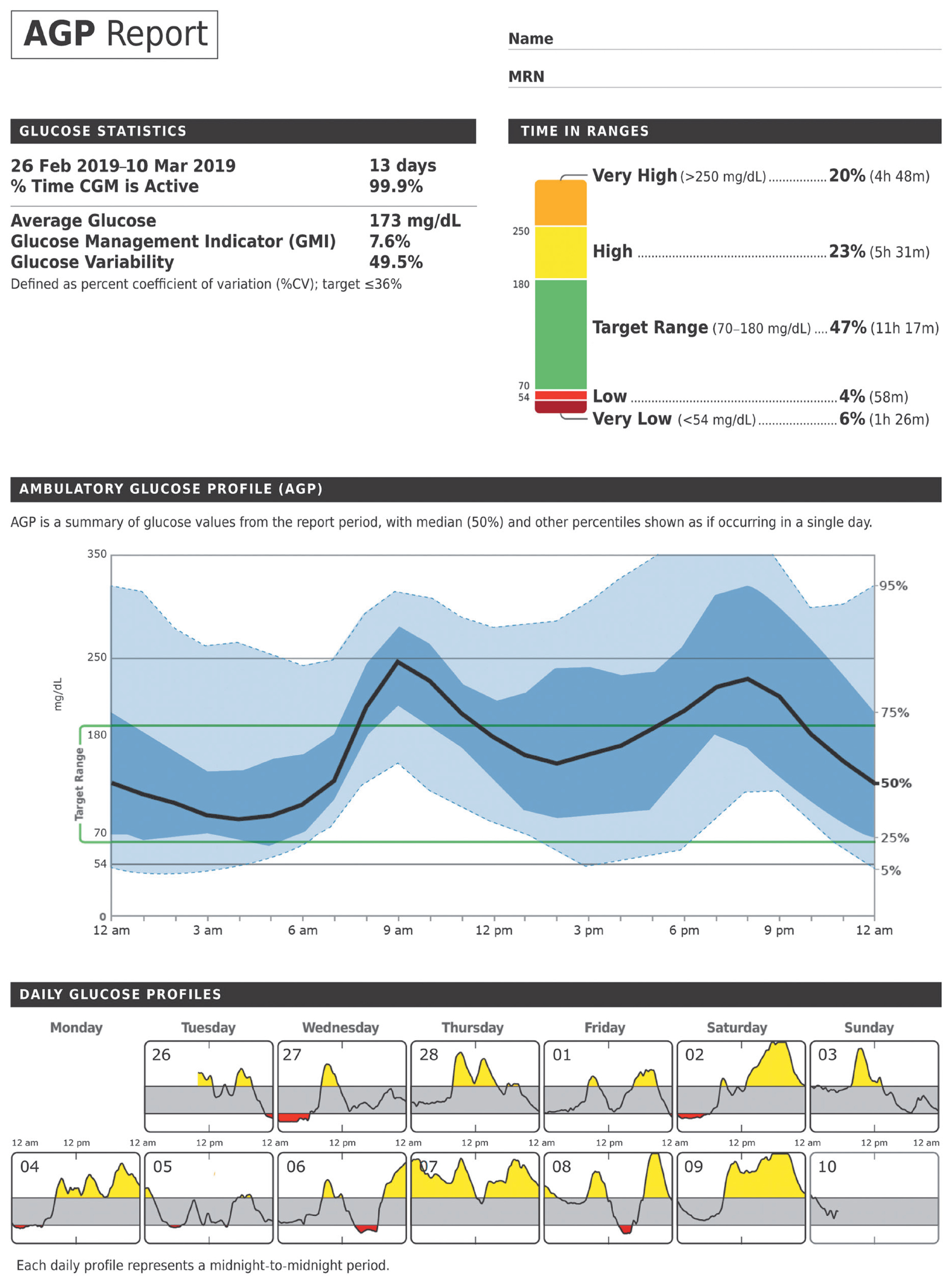
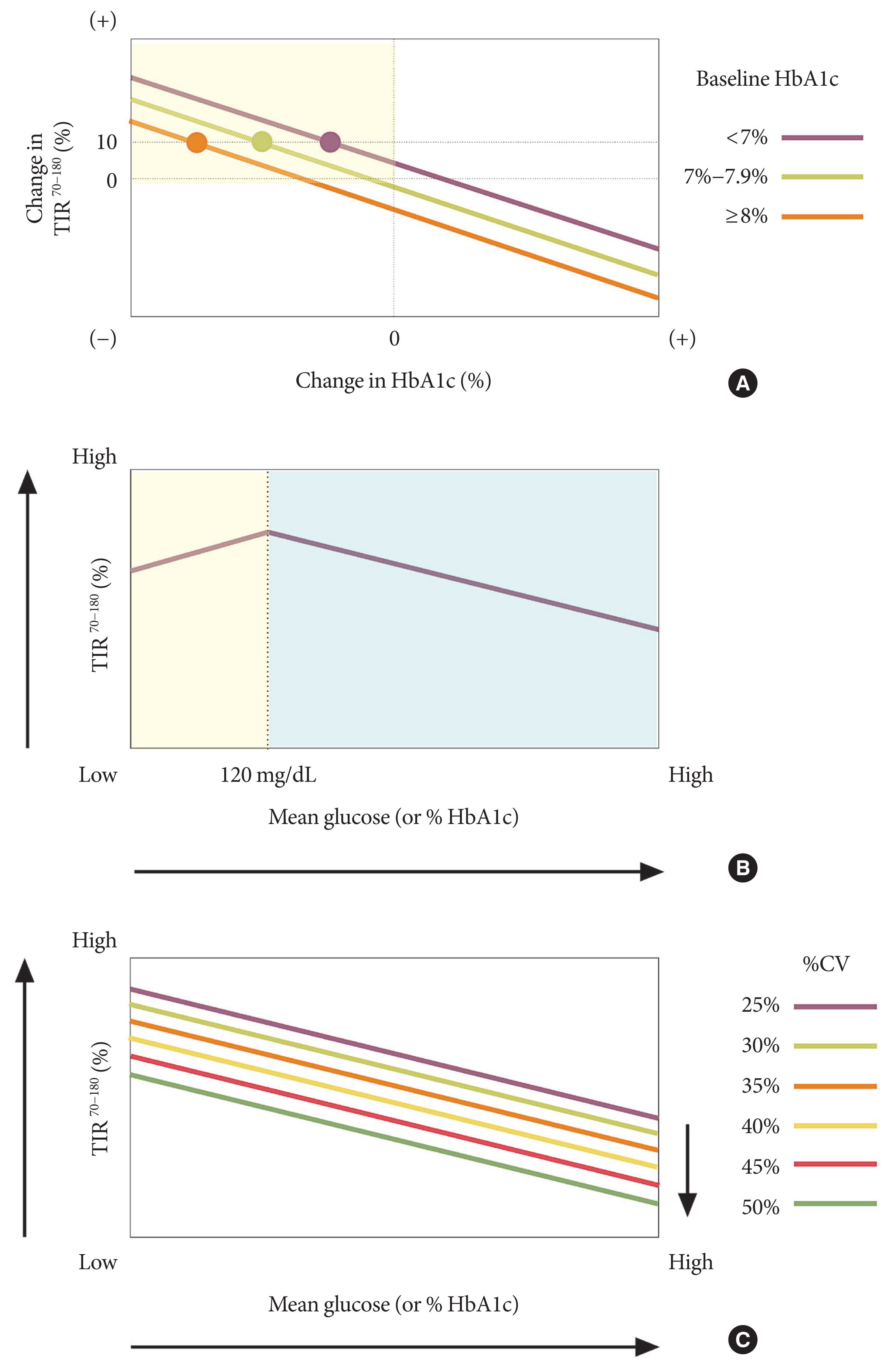
- Glycosylated hemoglobin (HbA1c) has been the sole surrogate marker for assessing diabetic complications. However, consistently reported limitations of HbA1c are that it lacks detailed information on short-term glycemic control and can be easily interfered with by various clinical conditions such as anemia, pregnancy, or liver disease. Thus, HbA1c alone may not represent the real glycemic status of a patient. The advancement of continuous glucose monitoring (CGM) has enabled both patients and healthcare providers to monitor glucose trends for a whole single day, which is not possible with HbA1c. This has allowed for the development of core metrics such as time spent in time in range (TIR), hyperglycemia, or hypoglycemia, and glycemic variability. Among the 10 core metrics, TIR is reported to represent overall glycemic control better than HbA1c alone. Moreover, various evidence supports TIR as a predictive marker of diabetes complications as well as HbA1c, as the inverse relationship between HbA1c and TIR reveals. However, there are more complex relationships between HbA1c, TIR, and other CGM metrics. This article provides information about 10 core metrics with particular focus on TIR and the relationships between the CGM metrics for comprehensive understanding of glycemic status using CGM.
Figure
Cited by 3 articles
-
Daytime Glycemic Variability and Frailty in Older Patients with Diabetes: a Pilot Study Using Continuous Glucose Monitoring
Seung Min Chung, Yun Hee Lee, Chang Oh Kim, Ji Yeon Lee, Sang-Man Jin, Seung-Hyun Yoo, Jun Sung Moon, Kwang Joon Kim
J Korean Med Sci. 2021;36(27):e190. doi: 10.3346/jkms.2021.36.e190.Glucose Profiles Assessed by Intermittently Scanned Continuous Glucose Monitoring System during the Perioperative Period of Metabolic Surgery
Kyuho Kim, Sung Hee Choi, Hak Chul Jang, Young Suk Park, Tae Jung Oh
Diabetes Metab J. 2022;46(5):713-721. doi: 10.4093/dmj.2021.0164.Advances in Continuous Glucose Monitoring and Integrated Devices for Management of Diabetes with Insulin-Based Therapy: Improvement in Glycemic Control
Jee Hee Yoo, Jae Hyeon Kim
Diabetes Metab J. 2023;47(1):27-41. doi: 10.4093/dmj.2022.0271.
Reference
-
1. Diabetes Control and Complications Trial Research Group. Nathan DM, Genuth S, Lachin J, Cleary P, Crofford O, Davis M, Rand L, Siebert C. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993; 329:977–86.
Article2. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998; 352:837–53.3. Beck RW, Connor CG, Mullen DM, Wesley DM, Bergenstal RM. The fallacy of average: how using HbA1c alone to assess glycemic control can be misleading. Diabetes Care. 2017; 40:994–9.
Article4. Suh S, Kim JH. Glycemic variability: how do we measure it and why is it important? Diabetes Metab J. 2015; 39:273–82.
Article5. Action to Control Cardiovascular Risk in Diabetes Study Group. Gerstein HC, Miller ME, Byington RP, Goff DC Jr, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH Jr, Probstfield JL, Simons-Morton DG, Friedewald WT. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008; 358:2545–59.6. Sheng CS, Tian J, Miao Y, Cheng Y, Yang Y, Reaven PD, Bloomgarden ZT, Ning G. Prognostic significance of long-term HbA1c variability for all-cause mortality in the ACCORD trial. Diabetes Care. 2020; 43:1185–90.
Article7. Kovatchev BP, Patek SD, Ortiz EA, Breton MD. Assessing sensor accuracy for non-adjunct use of continuous glucose monitoring. Diabetes Technol Ther. 2015; 17:177–86.
Article8. Rodbard D. Continuous glucose monitoring: a review of successes, challenges, and opportunities. Diabetes Technol Ther. 2016; 18(Suppl 2):S3–13.
Article9. Maiorino MI, Signoriello S, Maio A, Chiodini P, Bellastella G, Scappaticcio L, Longo M, Giugliano D, Esposito K. Effects of continuous glucose monitoring on metrics of glycemic control in diabetes: a systematic review with meta-analysis of randomized controlled trials. Diabetes Care. 2020; 43:1146–56.
Article10. Battelino T, Danne T, Bergenstal RM, Amiel SA, Beck R, Biester T, Bosi E, Buckingham BA, Cefalu WT, Close KL, Cobelli C, Dassau E, DeVries JH, Donaghue KC, Dovc K, Doyle FJ 3rd, Garg S, Grunberger G, Heller S, Heinemann L, Hirsch IB, Hovorka R, Jia W, Kordonouri O, Kovatchev B, Kowalski A, Laffel L, Levine B, Mayorov A, Mathieu C, Murphy HR, Nimri R, Norgaard K, Parkin CG, Renard E, Rodbard D, Saboo B, Schatz D, Stoner K, Urakami T, Weinzimer SA, Phillip M. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care. 2019; 42:1593–603.
Article11. Xing D, Kollman C, Beck RW, Tamborlane WV, Laffel L, Buckingham BA, Wilson DM, Weinzimer S, Fiallo-Scharer R, Ruedy KJ. Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. Optimal sampling intervals to assess long-term glycemic control using continuous glucose monitoring. Diabetes Technol Ther. 2011; 13:351–8.
Article12. Riddlesworth TD, Beck RW, Gal RL, Connor CG, Bergenstal RM, Lee S, Willi SM. Optimal sampling duration for continuous glucose monitoring to determine long-term glycemic control. Diabetes Technol Ther. 2018; 20:314–6.
Article13. Ambulatory Glucose Profile. AGP reports: CGM AGP Report (Continuous Glucose Monitor) New v4.0. Available from: http://agpreport.org/agp/agpreports(cited 2020 Nov 30).14. Bergenstal RM, Ahmann AJ, Bailey T, Beck RW, Bissen J, Buckingham B, Deeb L, Dolin RH, Garg SK, Goland R, Hirsch IB, Klonoff DC, Kruger DF, Matfin G, Mazze RS, Olson BA, Parkin C, Peters A, Powers MA, Rodriguez H, Southerland P, Strock ES, Tamborlane W, Wesley DM. Recommendations for standardizing glucose reporting and analysis to optimize clinical decision making in diabetes: the Ambulatory Glucose Profile (AGP). Diabetes Technol Ther. 2013; 15:198–211.
Article15. Bergenstal RM, Beck RW, Close KL, Grunberger G, Sacks DB, Kowalski A, Brown AS, Heinemann L, Aleppo G, Ryan DB, Riddlesworth TD, Cefalu WT. Glucose management indicator (GMI): a new term for estimating A1C from continuous glucose monitoring. Diabetes Care. 2018; 41:2275–80.
Article16. Aleppo G, Ruedy KJ, Riddlesworth TD, Kruger DF, Peters AL, Hirsch I, Bergenstal RM, Toschi E, Ahmann AJ, Shah VN, Rickels MR, Bode BW, Philis-Tsimikas A, Pop-Busui R, Rodriguez H, Eyth E, Bhargava A, Kollman C, Beck RW. REPLACE-BG Study Group. REPLACE-BG: a randomized trial comparing continuous glucose monitoring with and without routine blood glucose monitoring in adults with well-controlled type 1 diabetes. Diabetes Care. 2017; 40:538–45.
Article17. Beck RW, Riddlesworth T, Ruedy K, Ahmann A, Bergenstal R, Haller S, Kollman C, Kruger D, McGill JB, Polonsky W, Toschi E, Wolpert H, Price D. DIAMOND Study Group. Effect of continuous glucose monitoring on glycemic control in adults with type 1 diabetes using insulin injections: the DIAMOND randomized clinical trial. JAMA. 2017; 317:371–8.18. Beck RW, Riddlesworth TD, Ruedy K, Ahmann A, Haller S, Kruger D, McGill JB, Polonsky W, Price D, Aronoff S, Aronson R, Toschi E, Kollman C, Bergenstal R. DIAMOND Study Group. Continuous glucose monitoring versus usual care in patients with type 2 diabetes receiving multiple daily insulin injections: a randomized trial. Ann Intern Med. 2017; 167:365–74.19. Heinemann L, Freckmann G, Ehrmann D, Faber-Heinemann G, Guerra S, Waldenmaier D, Hermanns N. Real-time continuous glucose monitoring in adults with type 1 diabetes and impaired hypoglycaemia awareness or severe hypoglycaemia treated with multiple daily insulin injections (HypoDE): a multicentre, randomised controlled trial. Lancet. 2018; 391:1367–77.
Article20. Grimsmann JM, von Sengbusch S, Freff M, Ermer U, Placzek K, Danne T, Hammer E, Holl RW. DPV Initiative. Glucose management indicator based on sensor data and laboratory HbA1c in people with type 1 diabetes from the DPV database: differences by sensor type. Diabetes Care. 2020; 43:e111–2.21. Angellotti E, Muppavarapu S, Siegel RD, Pittas AG. The calculation of the glucose management indicator is influenced by the continuous glucose monitoring system and patient race. Diabetes Technol Ther. 2020; 22:651–7.
Article22. Beck RW, Bergenstal RM, Cheng P, Kollman C, Carlson AL, Johnson ML, Rodbard D. The relationships between time in range, hyperglycemia metrics, and HbA1c. J Diabetes Sci Technol. 2019; 13:614–26.
Article23. Vigersky RA, McMahon C. The relationship of hemoglobin A1C to time-in-range in patients with diabetes. Diabetes Technol Ther. 2019; 21:81–5.
Article24. Fabris C, Heinemann L, Beck R, Cobelli C, Kovatchev B. Estimation of hemoglobin A1c from continuous glucose monitoring data in individuals with type 1 diabetes: is time in range all we need? Diabetes Technol Ther. 2020; 22:501–8.
Article25. Bergenstal RM, Klonoff DC, Garg SK, Bode BW, Meredith M, Slover RH, Ahmann AJ, Welsh JB, Lee SW, Kaufman FR. AS-PIRE In-Home Study Group. Threshold-based insulin-pump interruption for reduction of hypoglycemia. N Engl J Med. 2013; 369:224–32.
Article26. Bergenstal RM, Garg S, Weinzimer SA, Buckingham BA, Bode BW, Tamborlane WV, Kaufman FR. Safety of a hybrid closed-loop insulin delivery system in patients with type 1 diabetes. JAMA. 2016; 316:1407–8.
Article27. Rodbard D. Glucose time in range, time above range, and time below range depend on mean or median glucose or HbA1c, glucose coefficient of variation, and shape of the glucose distribution. Diabetes Technol Ther. 2020; 22:492–500.
Article28. Lu J, Home PD, Zhou J. Comparison of multiple cut points for time in range in relation to risk of abnormal carotid intima-media thickness and diabetic retinopathy. Diabetes Care. 2020; 43:e99–101.
Article29. Lu J, Ma X, Shen Y, Wu Q, Wang R, Zhang L, Mo Y, Lu W, Zhu W, Bao Y, Vigersky RA, Jia W, Zhou J. Time in range is associated with carotid intima-media thickness in type 2 diabetes. Diabetes Technol Ther. 2020; 22:72–8.
Article30. Lu J, Ma X, Zhou J, Zhang L, Mo Y, Ying L, Lu W, Zhu W, Bao Y, Vigersky RA, Jia W. Association of time in range, as assessed by continuous glucose monitoring, with diabetic retinopathy in type 2 diabetes. Diabetes Care. 2018; 41:2370–6.
Article31. Ranjan AG, Rosenlund SV, Hansen TW, Rossing P, Andersen S, Norgaard K. Improved time in range over 1 year is associated with reduced albuminuria in individuals with sensor-augmented insulin pump-treated type 1 diabetes. Diabetes Care. 2020; 43:2882–5.32. Yang J, Yang X, Zhao D, Wang X, Wei W, Yuan H. Association of time in range, as assessed by continuous glucose monitoring, with painful diabetic polyneuropathy. J Diabetes Investig. 2020. Sep. 3. [Epub]. https://doi.org/10.1111/jdi.13394 .
Article33. Yoo JH, Choi MS, Ahn J, Park SW, Kim Y, Hur KY, Jin SM, Kim G, Kim JH. Association between continuous glucose monitoring-derived time in range, other core metrics, and albuminuria in type 2 diabetes. Diabetes Technol Ther. 2020; 22:768–76.
Article34. Beck RW, Bergenstal RM, Riddlesworth TD, Kollman C, Li Z, Brown AS, Close KL. Validation of time in range as an outcome measure for diabetes clinical trials. Diabetes Care. 2019; 42:400–5.
Article35. Rodbard D. Clinical interpretation of indices of quality of glycemic control and glycemic variability. Postgrad Med. 2011; 123:107–18.
Article36. Rodbard D. Hypo- and hyperglycemia in relation to the mean, standard deviation, coefficient of variation, and nature of the glucose distribution. Diabetes Technol Ther. 2012; 14:868–76.
Article37. Chon S. How can we easily measure glycemic variability in diabetes mellitus? Diabetes Metab J. 2015; 39:114–6.
Article38. Monnier L, Wojtusciszyn A, Molinari N, Colette C, Renard E, Owens D. Respective contributions of glycemic variability and mean daily glucose as predictors of hypoglycemia in type 1 diabetes: are they equivalent? Diabetes Care. 2020; 43:821–7.
Article39. Kovatchev B, Cobelli C. Glucose variability: timing, risk analysis, and relationship to hypoglycemia in diabetes. Diabetes Care. 2016; 39:502–10.
Article40. Jin SM, Kim TH, Bae JC, Hur KY, Lee MS, Lee MK, Kim JH. Clinical factors associated with absolute and relative measures of glycemic variability determined by continuous glucose monitoring: an analysis of 480 subjects. Diabetes Res Clin Pract. 2014; 104:266–72.
Article41. Monnier L, Colette C, Wojtusciszyn A, Dejager S, Renard E, Molinari N, Owens DR. Toward defining the threshold between low and high glucose variability in diabetes. Diabetes Care. 2017; 40:832–8.
Article42. Cryer PE. Individualized glycemic goals and an expanded classification of severe hypoglycemia in diabetes. Diabetes Care. 2017; 40:1641–3.
Article43. Jun JE, Jin SM, Baek J, Oh S, Hur KY, Lee MS, Lee MK, Kim JH. The association between glycemic variability and diabetic cardiovascular autonomic neuropathy in patients with type 2 diabetes. Cardiovasc Diabetol. 2015; 14:70.
Article44. Jun JE, Lee SE, Lee YB, Ahn JY, Kim G, Hur KY, Lee MK, Jin SM, Kim JH. Continuous glucose monitoring defined glucose variability is associated with cardiovascular autonomic neuropathy in type 1 diabetes. Diabetes Metab Res Rev. 2019; 35:e3092.
Article45. Matsutani D, Sakamoto M, Iuchi H, Minato S, Suzuki H, Kayama Y, Takeda N, Horiuchi R, Utsunomiya K. Glycemic variability in continuous glucose monitoring is inversely associated with baroreflex sensitivity in type 2 diabetes: a preliminary report. Cardiovasc Diabetol. 2018; 17:36.
Article46. Ha WC, Oh SJ, Kim JH, Lee JM, Chang SA, Sohn TS, Son HS. Severe hypoglycemia is a serious complication and becoming an economic burden in diabetes. Diabetes Metab J. 2012; 36:280–4.
Article47. Rama Chandran S, Tay WL, Lye WK, Lim LL, Ratnasingam J, Tan AT, Gardner DS. Beyond HbA1c: comparing glycemic variability and glycemic indices in predicting hypoglycemia in type 1 and type 2 diabetes. Diabetes Technol Ther. 2018; 20:353–62.
Article48. Park C, Le QA. The effectiveness of continuous glucose monitoring in patients with type 2 diabetes: a systematic review of literature and meta-analysis. Diabetes Technol Ther. 2018; 20:613–21.
Article49. Lee SH, Min KW, Lee BW, Jeong IK, Yoo SJ, Kwon HS, Choi YH, Yoon KH. Effect of dapagliflozin as an add-on therapy to insulin on the glycemic variability in subjects with type 2 diabetes mellitus (DIVE): a multicenter, placebo-controlled, double-blind, randomized study. Diabetes Metab J. 2020. May. 28. [Epub]. https://doi.org/10.4093/dmj.2019.0203 .
Article50. Kim G, Lim S, Kwon HS, Park IB, Ahn KJ, Park CY, Kwon SK, Kim HS, Park SW, Kim SG, Moon MK, Kim ES, Chung CH, Park KS, Kim M, Chung DJ, Lee CB, Kim TH, Lee MK. Efficacy and safety of evogliptin treatment in patients with type 2 diabetes: a multicentre, active-controlled, randomized, double-blind study with open-label extension (the EVERGREEN study). Diabetes Obes Metab. 2020; 22:1527–36.51. Kwak SH, Hwang YC, Won JC, Bae JC, Kim HJ, Suh S, Lee EY, Lee S, Kim SY, Kim JH. Comparison of the effects of gemigliptin and dapagliflozin on glycaemic variability in type 2 diabetes: a randomized, open-label, active-controlled, 12-week study (STABLE II study). Diabetes Obes Metab. 2020; 22:173–81.
Article52. Fonda SJ, Salkind SJ, Walker MS, Chellappa M, Ehrhardt N, Vigersky RA. Heterogeneity of responses to real-time continuous glucose monitoring (RT-CGM) in patients with type 2 diabetes and its implications for application. Diabetes Care. 2013; 36:786–92.
Article53. Vigersky R, Shrivastav M. Role of continuous glucose monitoring for type 2 in diabetes management and research. J Diabetes Complications. 2017; 31:280–7.
Article54. Gehlaut RR, Dogbey GY, Schwartz FL, Marling CR, Shubrook JH. Hypoglycemia in type 2 diabetes: more common than you think: a continuous glucose monitoring study. J Diabetes Sci Technol. 2015; 9:999–1005.55. Schrangl P, Reiterer F, Heinemann L, Freckmann G, Del Re L. Limits to the evaluation of the accuracy of continuous glucose monitoring systems by clinical trials. Biosensors (Basel). 2018; 8:50.
Article56. Messer LH, Tanenbaum ML, Cook PF, Wong JJ, Hanes SJ, Driscoll KA, Hood KK. Cost, hassle, and on-body experience: barriers to diabetes device use in adolescents and potential intervention targets. Diabetes Technol Ther. 2020; 22:760–7.
Article57. Wong JC, Foster NC, Maahs DM, Raghinaru D, Bergenstal RM, Ahmann AJ, Peters AL, Bode BW, Aleppo G, Hirsch IB, Kleis L, Chase HP, DuBose SN, Miller KM, Beck RW, Adi S. T1D Exchange Clinic Network. Real-time continuous glucose monitoring among participants in the T1D Exchange clinic registry. Diabetes Care. 2014; 37:2702–9.
Article58. Haak T, Hanaire H, Ajjan R, Hermanns N, Riveline JP, Rayman G. Flash glucose-sensing technology as a replacement for blood glucose monitoring for the management of insulin-treated type 2 diabetes: a multicenter, open-label randomized controlled trial. Diabetes Ther. 2017; 8:55–73.
Article59. Oskarsson P, Antuna R, Geelhoed-Duijvestijn P, Kroger J, Weitgasser R, Bolinder J. Impact of flash glucose monitoring on hypoglycaemia in adults with type 1 diabetes managed with multiple daily injection therapy: a pre-specified subgroup analysis of the IMPACT randomised controlled trial. Diabetologia. 2018; 61:539–50.
Article60. Bolinder J, Antuna R, Geelhoed-Duijvestijn P, Kroger J, Weitgasser R. Novel glucose-sensing technology and hypoglycaemia in type 1 diabetes: a multicentre, non-masked, randomised controlled trial. Lancet. 2016; 388:2254–63.
Article61. Hermanns N, Ehrmann D, Schipfer M, Kroger J, Haak T, Kulzer B. The impact of a structured education and treatment programme (FLASH) for people with diabetes using a flash sensor-based glucose monitoring system: results of a randomized controlled trial. Diabetes Res Clin Pract. 2019; 150:111–21.
Article62. Yaron M, Roitman E, Aharon-Hananel G, Landau Z, Ganz T, Yanuv I, Rozenberg A, Karp M, Ish-Shalom M, Singer J, Wainstein J, Raz I. Effect of flash glucose monitoring technology on glycemic control and treatment satisfaction in patients with type 2 diabetes. Diabetes Care. 2019; 42:1178–84.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Time in Range from Continuous Glucose Monitoring: A Novel Metric for Glycemic Control
- Application of Continuous Glucose Monitoring System (CGMS) and Patient Education
- Importance of continuous glucose monitoring in the treatment of diabetes mellitus
- Use of Flash Glucose Monitoring in Patients on Intensive Insulin Treatment
- Advances in Glucose Monitoring and Insulin Therapy in Intensive Care Unit Patients