Consequences of Obesity on the Sense of Taste: Taste Buds as Treatment Targets?
- Affiliations
-
- 1Helmholtz Institute for Metabolic, Obesity and Vascular Research (HI-MAG) of the Helmholtz Center Munich at the University of Leipzig and University Hospital Leipzig, Leipzig, Germany.
- 2Medical Department III (Endocrinology, Nephrology and Rheumatology), University of Leipzig, Leipzig, Germany.
- KMID: 2513011
- DOI: http://doi.org/10.4093/dmj.2020.0058
Abstract
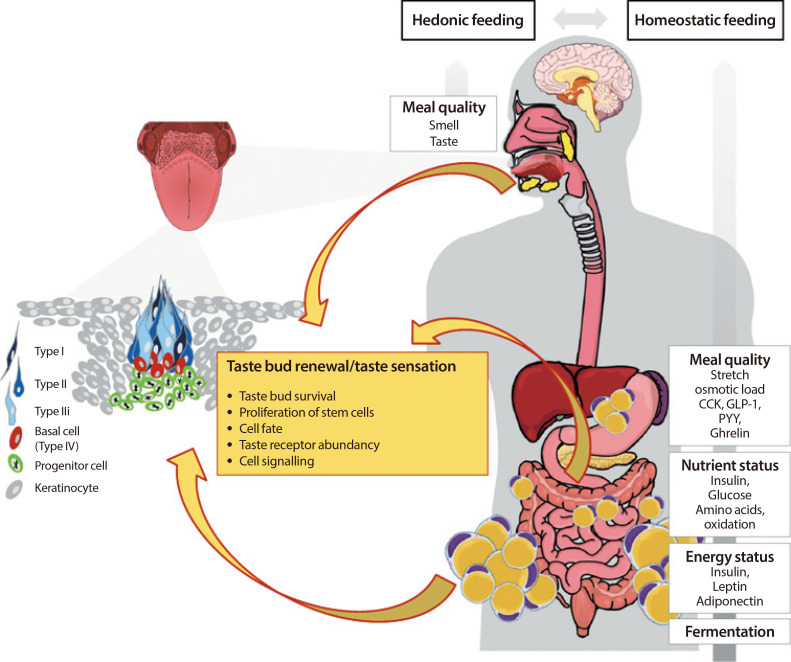
Premature obesity-related mortality is caused by cardiovascular and pulmonary diseases, type 2 diabetes mellitus, physical disabilities, osteoarthritis, and certain types of cancer. Obesity is caused by a positive energy balance due to hyper-caloric nutrition, low physical activity, and energy expenditure. Overeating is partially driven by impaired homeostatic feedback of the peripheral energy status in obesity. However, food with its different qualities is a key driver for the reward driven hedonic feeding with tremendous consequences on calorie consumption. In addition to visual and olfactory cues, taste buds of the oral cavity process the earliest signals which affect the regulation of food intake, appetite and satiety. Therefore, taste buds may play a crucial role how food related signals are transmitted to the brain, particularly in priming the body for digestion during the cephalic phase. Indeed, obesity development is associated with a significant reduction in taste buds. Impaired taste bud sensitivity may play a causal role in the pathophysiology of obesity in children and adolescents. In addition, genetic variation in taste receptors has been linked to body weight regulation. This review discusses the importance of taste buds as contributing factors in the development of obesity and how obesity may affect the sense of taste, alterations in food preferences and eating behavior.
Keyword
Figure
Cited by 2 articles
-
Food Preferences and Obesity
Sara Spinelli, Erminio Monteleone
Endocrinol Metab. 2021;36(2):209-219. doi: 10.3803/EnM.2021.105.Recent Advances in Understanding Peripheral Taste Decoding I: 2010 to 2020
Jea Hwa Jang, Obin Kwon, Seok Jun Moon, Yong Taek Jeong
Endocrinol Metab. 2021;36(3):469-477. doi: 10.3803/EnM.2021.302.
Reference
-
1. Blüher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol. 2019; 15:288–298. PMID: 30814686.
Article2. Rossi MA, Stuber GD. Overlapping brain circuits for homeostatic and hedonic feeding. Cell Metab. 2018; 27:42–56. PMID: 29107504.
Article3. Archer N, Shaw J, Cochet-Broch M, Bunch R, Poelman A, Barendse W, Duesing K. Obesity is associated with altered gene expression in human tastebuds. Int J Obes (Lond). 2019; 43:1475–1484. PMID: 30696932.
Article4. Hardikar S, Hochenberger R, Villringer A, Ohla K. Higher sensitivity to sweet and salty taste in obese compared to lean individuals. Appetite. 2017; 111:158–165. PMID: 27988366.
Article5. Kittrell H, Graber W, Mariani E, Czaja K, Hajnal A, Di Lorenzo PM. Taste and odor preferences following Roux-en-Y surgery in humans. PLoS One. 2018; 13:e0199508. PMID: 29975712.
Article6. Skrandies W, Zschieschang R. Olfactory and gustatory functions and its relation to body weight. Physiol Behav. 2015; 142:1–4. PMID: 25619950.
Article7. Tichansky DS, Glatt AR, Madan AK, Harper J, Tokita K, Boughter JD. Decrease in sweet taste in rats after gastric bypass surgery. Surg Endosc. 2011; 25:1176–1181. PMID: 20844896.
Article8. Liu D, Archer N, Duesing K, Hannan G, Keast R. Mechanism of fat taste perception: association with diet and obesity. Prog Lipid Res. 2016; 63:41–49. PMID: 27155595.
Article9. Kaufman A, Choo E, Koh A, Dando R. Inflammation arising from obesity reduces taste bud abundance and inhibits renewal. PLoS Biol. 2018; 16:e2001959. PMID: 29558472.
Article10. Kubasova N, Burdakov D, Domingos AI. Sweet and low on leptin: hormonal regulation of sweet taste buds. Diabetes. 2015; 64:3651–3652. PMID: 26494218.11. Meredith TL, Corcoran A, Roper SD. Leptin's effect on taste bud calcium responses and transmitter secretion. Chem Senses. 2015; 40:217–222. PMID: 25537017.
Article12. Garcia-Bailo B, Toguri C, Eny KM, El-Sohemy A. Genetic variation in taste and its influence on food selection. OMICS. 2009; 13:69–80. PMID: 18687042.
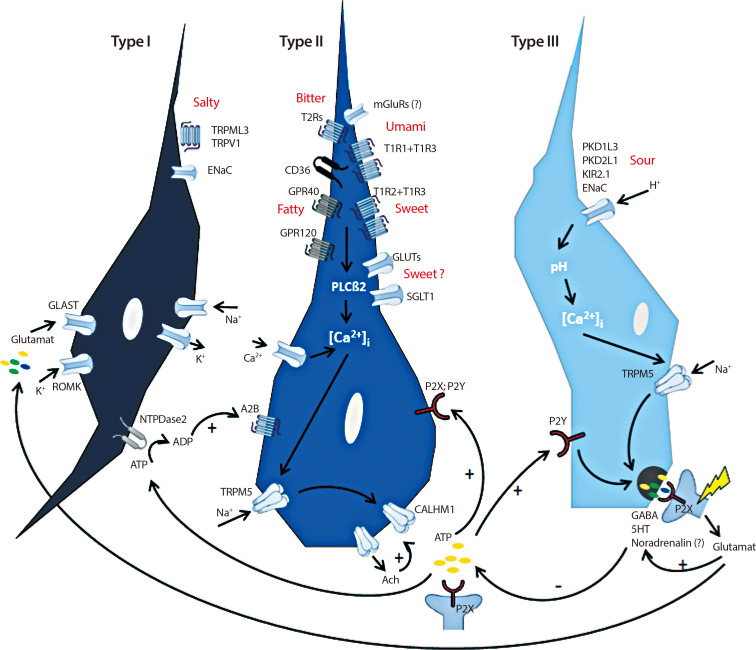
Article13. Chandrashekar J, Hoon MA, Ryba NJ, Zuker CS. The receptors and cells for mammalian taste. Nature. 2006; 444:288–294. PMID: 17108952.
Article14. Besnard P, Passilly-Degrace P, Khan NA. Taste of fat: a sixth taste modality? Physiol Rev. 2016; 96:151–176. PMID: 26631596.
Article15. Roper SD, Chaudhari N. Taste buds: cells, signals and synapses. Nat Rev Neurosci. 2017; 18:485–497. PMID: 28655883.
Article16. Travers SP, Nicklas K. Taste bud distribution in the rat pharynx and larynx. Anat Rec. 1990; 227:373–379. PMID: 2372140.
Article17. Nilsson B. The occurrence of taste buds in the palate of human adults as evidenced by light microscopy. Acta Odontol Scand. 1979; 37:253–258. PMID: 294117.
Article18. Loper HB, La Sala M, Dotson C, Steinle N. Taste perception, associated hormonal modulation, and nutrient intake. Nutr Rev. 2015; 73:83–91.
Article19. Gravina SA, Yep GL, Khan M. Human biology of taste. Ann Saudi Med. 2013; 33:217–222. PMID: 23793421.
Article20. Calvo SS, Egan JM. The endocrinology of taste receptors. Nat Rev Endocrinol. 2015; 11:213–227. PMID: 25707779.
Article21. Murray RG. Cellular relations in mouse circumvallate taste buds. Microsc Res Tech. 1993; 26:209–224. PMID: 8241560.
Article22. Vandenbeuch A, Anderson CB, Parnes J, Enjyoji K, Robson SC, Finger TE, Kinnamon SC. Role of the ectonucleotidase NTPDase2 in taste bud function. Proc Natl Acad Sci U S A. 2013; 110:14789–14794. PMID: 23959882.
Article23. Lawton DM, Furness DN, Lindemann B, Hackney CM. Localization of the glutamate-aspartate transporter, GLAST, in rat taste buds. Eur J Neurosci. 2000; 12:3163–3171. PMID: 10998100.
Article24. Dvoryanchikov G, Sinclair MS, Perea-Martinez I, Wang T, Chaudhari N. Inward rectifier channel, ROMK, is localized to the apical tips of glial-like cells in mouse taste buds. J Comp Neurol. 2009; 517:1–14. PMID: 19708028.
Article25. Tomchik SM, Berg S, Kim JW, Chaudhari N, Roper SD. Breadth of tuning and taste coding in mammalian taste buds. Version 2. J Neurosci. 2007; 27:10840–10848. PMID: 17913917.26. Ozeck M, Brust P, Xu H, Servant G. Receptors for bitter, sweet and umami taste couple to inhibitory G protein signaling pathways. Eur J Pharmacol. 2004; 489:139–149. PMID: 15087236.
Article27. Toyono T, Seta Y, Kataoka S, Oda M, Toyoshima K. Differential expression of the glucose transporters in mouse gustatory papillae. Cell Tissue Res. 2011; 345:243–252. PMID: 21739243.
Article28. Yee KK, Sukumaran SK, Kotha R, Gilbertson TA, Margolskee RF. Glucose transporters and ATP-gated K+ (KATP) metabolic sensors are present in type 1 taste receptor 3 (T1r3)-expressing taste cells. Proc Natl Acad Sci U S A. 2011; 108:5431–5436. PMID: 21383163.29. Nelson G, Chandrashekar J, Hoon MA, Feng L, Zhao G, Ryba NJ, Zuker CS. An amino-acid taste receptor. Nature. 2002; 416:199–202. PMID: 11894099.
Article30. Zhao GQ, Zhang Y, Hoon MA, Chandrashekar J, Erlenbach I, Ryba NJ, Zuker CS. The receptors for mammalian sweet and umami taste. Cell. 2003; 115:255–266. PMID: 14636554.
Article31. Chaudhari N, Landin AM, Roper SD. A metabotropic glutamate receptor variant functions as a taste receptor. Nat Neurosci. 2000; 3:113–119. PMID: 10649565.
Article32. Behrens M, Foerster S, Staehler F, Raguse JD, Meyerhof W. Gustatory expression pattern of the human TAS2R bitter receptor gene family reveals a heterogenous population of bitter responsive taste receptor cells. J Neurosci. 2007; 27:12630–12640. PMID: 18003842.
Article33. Adler E, Hoon MA, Mueller KL, Chandrashekar J, Ryba NJ, Zuker CS. A novel family of mammalian taste receptors. Cell. 2000; 100:693–702. PMID: 10761934.
Article34. Liu D, Liman ER. Intracellular Ca2+ and the phospholipid PIP2 regulate the taste transduction ion channel TRPM5. Proc Natl Acad Sci U S A. 2003; 100:15160–15165. PMID: 14657398.35. Taruno A, Vingtdeux V, Ohmoto M, Ma Z, Dvoryanchikov G, Li A, Adrien L, Zhao H, Leung S, Abernethy M, Koppel J, Davies P, Civan MM, Chaudhari N, Matsumoto I, Hellekant G, Tordoff MG, Marambaud P, Foskett JK. CALHM1 ion channel mediates purinergic neurotransmission of sweet, bitter and umami tastes. Nature. 2013; 495:223–226. PMID: 23467090.
Article36. Kinnamon SC, Finger TE. A taste for ATP: neurotransmission in taste buds. Front Cell Neurosci. 2013; 7:264. PMID: 24385952.
Article37. Huang YJ, Maruyama Y, Dvoryanchikov G, Pereira E, Chaudhari N, Roper SD. The role of pannexin 1 hemichannels in ATP release and cell-cell communication in mouse taste buds. Proc Natl Acad Sci U S A. 2007; 104:6436–6441. PMID: 17389364.
Article38. Dando R, Roper SD. Acetylcholine is released from taste cells, enhancing taste signalling. J Physiol. 2012; 590:3009–3017. PMID: 22570381.
Article39. Dando R, Dvoryanchikov G, Pereira E, Chaudhari N, Roper SD. Adenosine enhances sweet taste through A2B receptors in the taste bud. Version 2. J Neurosci. 2012; 32:322–330. PMID: 22219293.40. Wilson CE, Finger TE, Kinnamon SC. Type III cells in anterior taste fields are more immunohistochemically diverse than those of posterior taste fields in mice. Chem Senses. 2017; 42:759–767. PMID: 28968659.
Article41. Lyall V, Alam RI, Phan DQ, Ereso GL, Phan TH, Malik SA, Montrose MH, Chu S, Heck GL, Feldman GM, DeSimone JA. Decrease in rat taste receptor cell intracellular pH is the proximate stimulus in sour taste transduction. Am J Physiol Cell Physiol. 2001; 281:C1005–C1013. PMID: 11502578.
Article42. Ye W, Chang RB, Bushman JD, Tu YH, Mulhall EM, Wilson CE, Cooper AJ, Chick WS, Hill-Eubanks DC, Nelson MT, Kinnamon SC, Liman ER. The K+ channel KIR2.1 functions in tandem with proton influx to mediate sour taste transduction. Proc Natl Acad Sci U S A. 2016; 113:E229–E238. PMID: 26627720.43. Huang AL, Chen X, Hoon MA, Chandrashekar J, Guo W, Trankner D, Ryba NJ, Zuker CS. The cells and logic for mammalian sour taste detection. Nature. 2006; 442:934–938. PMID: 16929298.
Article44. Huque T, Cowart BJ, Dankulich-Nagrudny L, Pribitkin EA, Bayley DL, Spielman AI, Feldman RS, Mackler SA, Brand JG. Sour ageusia in two individuals implicates ion channels of the ASIC and PKD families in human sour taste perception at the anterior tongue. PLoS One. 2009; 4:e7347. PMID: 19812697.
Article45. Gilbertson TA, Avenet P, Kinnamon SC, Roper SD. Proton currents through amiloride-sensitive Na channels in hamster taste cells. Role in acid transduction. J Gen Physiol. 1992; 100:803–824. PMID: 1335477.
Article46. Heck GL, Mierson S, DeSimone JA. Salt taste transduction occurs through an amiloride-sensitive sodium transport pathway. Science. 1984; 223:403–405. PMID: 6691151.
Article47. Bachmanov AA, Bosak NP, Lin C, Matsumoto I, Ohmoto M, Reed DR, Nelson TM. Genetics of taste receptors. Curr Pharm Des. 2014; 20:2669–2683. PMID: 23886383.
Article48. Martin C, Passilly-Degrace P, Gaillard D, Merlin JF, Chevrot M, Besnard P. The lipid-sensor candidates CD36 and GPR120 are differentially regulated by dietary lipids in mouse taste buds: impact on spontaneous fat preference. PloS One. 2011; 6:e24014. PMID: 21901153.
Article49. Niot I, Besnard P. Appetite control by the tongue-gut axis and evaluation of the role of CD36/SR-B2. Biochimie. 2017; 136:27–32. PMID: 28238842.
Article50. Laugerette F, Passilly-Degrace P, Patris B, Niot I, Febbraio M, Montmayeur JP, Besnard P. CD36 involvement in orosensory detection of dietary lipids, spontaneous fat preference, and digestive secretions. J Clin Invest. 2005; 115:3177–3184. PMID: 16276419.
Article51. Zocchi D, Wennemuth G, Oka Y. The cellular mechanism for water detection in the mammalian taste system. Nat Neurosci. 2017; 20:927–933. PMID: 28553944.
Article52. Ohsu T, Amino Y, Nagasaki H, Yamanaka T, Takeshita S, Hatanaka T, Maruyama Y, Miyamura N, Eto Y. Involvement of the calcium-sensing receptor in human taste perception. J Biol Chem. 2010; 285:1016–1022. PMID: 19892707.
Article53. Mistretta CM, Kumari A. Hedgehog signaling regulates taste organs and oral sensation: distinctive roles in the epithelium, stroma, and innervation. Int J Mol Sci. 2019; 20:1341.
Article54. Lee H, Macpherson LJ, Parada CA, Zuker CS, Ryba NJP. Rewiring the taste system. Nature. 2017; 548:330–333. PMID: 28792937.
Article55. Chikazoe J, Lee DH, Kriegeskorte N, Anderson AK. Distinct representations of basic taste qualities in human gustatory cortex. Nat Commun. 2019; 10:1048. PMID: 30837463.
Article56. Barlow LA. Progress and renewal in gustation: new insights into taste bud development. Development. 2015; 142:3620–3629. PMID: 26534983.
Article57. Dyer JS, Rosenfeld CR. Metabolic imprinting by prenatal, perinatal, and postnatal overnutrition: a review. Semin Reprod Med. 2011; 29:266–276. PMID: 21769766.
Article58. Barlow LA, Klein OD. Developing and regenerating a sense of taste. Curr Top Dev Biol. 2015; 111:401–419. PMID: 25662267.
Article59. Liu HX, Ermilov A, Grachtchouk M, Li L, Gumucio DL, Dlugosz AA, Mistretta CM. Multiple Shh signaling centers participate in fungiform papilla and taste bud formation and maintenance. Dev Biol. 2013; 382:82–97. PMID: 23916850.
Article60. Liu F, Thirumangalathu S, Gallant NM, Yang SH, Stoick-Cooper CL, Reddy ST, Andl T, Taketo MM, Dlugosz AA, Moon RT, Barlow LA, Millar SE. Wnt-beta-catenin signaling initiates taste papilla development. Nat Genet. 2007; 39:106–112. PMID: 17128274.61. Rothova M, Thompson H, Lickert H, Tucker AS. Lineage tracing of the endoderm during oral development. Dev Dyn. 2012; 241:1183–1191. PMID: 22581563.
Article62. Thirumangalathu S, Harlow DE, Driskell AL, Krimm RF, Barlow LA. Fate mapping of mammalian embryonic taste bud progenitors. Development. 2009; 136:1519–1528. PMID: 19363153.
Article63. Feng P, Huang L, Wang H. Taste bud homeostasis in health, disease, and aging. Chem Senses. 2014; 39:3–16. PMID: 24287552.
Article64. Miura H, Barlow LA. Taste bud regeneration and the search for taste progenitor cells. Arch Ital Biol. 2010; 148:107–118. PMID: 20830973.65. Miura H, Kusakabe Y, Harada S. Cell lineage and differentiation in taste buds. Arch Histol Cytol. 2006; 69:209–225. PMID: 17287576.
Article66. Matsumoto I, Ohmoto M, Narukawa M, Yoshihara Y, Abe K. Skn-1a (Pou2f3) specifies taste receptor cell lineage. Nat Neurosci. 2011; 14:685–687. PMID: 21572433.
Article67. Castillo-Azofeifa D, Seidel K, Gross L, Golden EJ, Jacquez B, Klein OD, Barlow LA. SOX2 regulation by hedgehog signaling controls adult lingual epithelium homeostasis. Development. 2018; 145:dev164889. PMID: 29945863.
Article68. Qin Y, Sukumaran SK, Jyotaki M, Redding K, Jiang P, Margolskee RF. Gli3 is a negative regulator of Tas1r3-expressing taste cells. PLoS Genet. 2018; 14:e1007058. PMID: 29415007.
Article69. Thirumangalathu S, Barlow LA. β-Catenin signaling regulates temporally discrete phases of anterior taste bud development. Development. 2015; 142:4309–4317. PMID: 26525674.
Article70. Kershaw JC, Mattes RD. Nutrition and taste and smell dysfunction. World J Otorhinolaryngol Head Neck Surg. 2018; 4:3–10. PMID: 30035256.
Article71. Woschnagg H, Stollberger C, Finsterer J. Loss of taste is loss of weight. Lancet. 2002; 359:891. PMID: 11897315.
Article72. Sartor F, Donaldson LF, Markland DA, Loveday H, Jackson MJ, Kubis HP. Taste perception and implicit attitude toward sweet related to body mass index and soft drink supplementation. Appetite. 2011; 57:237–246. PMID: 21600942.
Article73. Pepino MY, Finkbeiner S, Beauchamp GK, Mennella JA. Obese women have lower monosodium glutamate taste sensitivity and prefer higher concentrations than do normal-weight women. Obesity (Silver Spring). 2010; 18:959–965. PMID: 20075854.
Article74. Gaillard D, Stratford JM. Measurement of behavioral taste responses in mice: two-bottle preference, lickometer, and conditioned taste-aversion tests. Curr Protoc Mouse Biol. 2016; 6:380–407. PMID: 27906463.
Article75. Hajnal A, Kovacs P, Ahmed T, Meirelles K, Lynch CJ, Cooney RN. Gastric bypass surgery alters behavioral and neural taste functions for sweet taste in obese rats. Am J Physiol Gastrointest Liver Physiol. 2010; 299:G967–G979. PMID: 20634436.
Article76. Nielsen MS, Andersen INSK, Lange B, Ritz C, le Roux CW, Schmidt JB, Sjodin A, Bredie WLP. Bariatric surgery leads to short-term effects on sweet taste sensitivity and hedonic evaluation of fatty food stimuli. Obesity (Silver Spring). 2019; 27:1796–1804. PMID: 31556242.
Article77. Sclafani A, Koopmans HS. Intestinal bypass surgery produces conditioned taste aversion in rats. Int J Obes. 1981; 5:497–500. PMID: 7309332.78. Wilson-Perez HE, Chambers AP, Sandoval DA, Stefater MA, Woods SC, Benoit SC, Seeley RJ. The effect of vertical sleeve gastrectomy on food choice in rats. Int J Obes (Lond). 2013; 37:288–295. PMID: 22334194.79. Mathes CM. Taste- and flavor-guided behaviors following Roux-en-Y gastric bypass in rodent models. Appetite. 2020; 146:104422. PMID: 31472198.
Article80. Mathes CM, Bueter M, Smith KR, Lutz TA, le Roux CW, Spector AC. Roux-en-Y gastric bypass in rats increases sucrose taste-related motivated behavior independent of pharmacological GLP-1-receptor modulation. Am J Physiol Regul Integr Comp Physiol. 2012; 302:R751–R767. PMID: 22170618.
Article81. Shoar S, Naderan M, Shoar N, Modukuru VR, Mahmoodzadeh H. Alteration pattern of taste perception after bariatric surgery: a systematic review of four taste domains. Obes Surg. 2019; 29:1542–1550. PMID: 30712168.
Article82. Makaronidis JM, Batterham RL. Potential mechanisms mediating sustained weight loss following roux-en-y gastric bypass and sleeve gastrectomy. Endocrinol Metab Clin North Am. 2016; 45:539–552. PMID: 27519129.
Article83. Makaronidis JM, Neilson S, Cheung WH, Tymoszuk U, Pucci A, Finer N, Doyle J, Hashemi M, Elkalaawy M, Adamo M, Jenkinson A, Batterham RL. Reported appetite, taste and smell changes following Roux-en-Y gastric bypass and sleeve gastrectomy: effect of gender, type 2 diabetes and relationship to post-operative weight loss. Appetite. 2016; 107:93–105. PMID: 27453553.
Article84. Van Vuuren MAJ, Strodl E, White KM, Lockie PD. Taste, enjoyment, and desire of flavors change after sleeve gastrectomy-short term results. Obes Surg. 2017; 27:1466–1473. PMID: 27981459.
Article85. Burge JC, Schaumburg JZ, Choban PS, DiSilvestro RA, Flancbaum L. Changes in patients' taste acuity after Roux-en-Y gastric bypass for clinically severe obesity. J Am Diet Assoc. 1995; 95:666–670. PMID: 7759742.
Article86. Holinski F, Menenakos C, Haber G, Olze H, Ordemann J. Olfactory and gustatory function after bariatric surgery. Obes Surg. 2015; 25:2314–2320. PMID: 25910980.
Article87. Altun H, Hanci D, Altun H, Batman B, Serin RK, Karip AB, Akyuz U. Improved gustatory sensitivity in morbidly obese patients after laparoscopic sleeve gastrectomy. Ann Otol Rhinol Laryngol. 2016; 125:536–540. PMID: 26848035.
Article88. Scruggs DM, Buffington C, Cowan GS Jr. Taste acuity of the morbidly obese before and after gastric bypass surgery. Obes Surg. 1994; 4:24–28. PMID: 10742759.
Article89. Zakeri R, Batterham RL. Potential mechanisms underlying the effect of bariatric surgery on eating behaviour. Curr Opin Endocrinol Diabetes Obes. 2018; 25:3–11. PMID: 29120924.
Article90. Umabiki M, Tsuzaki K, Kotani K, Nagai N, Sano Y, Matsuoka Y, Kitaoka K, Okami Y, Sakane N, Higashi A. The improvement of sweet taste sensitivity with decrease in serum leptin levels during weight loss in obese females. Tohoku J Exp Med. 2010; 220:267–271. PMID: 20383037.
Article91. Olea Lopez AL, Johnson L. Associations between restrained eating and the size and frequency of overall intake, meal, snack and drink occasions in the UK adult national diet and nutrition survey. PLoS One. 2016; 11:e0156320. PMID: 27227409.
Article92. Stunkard AJ, Messick S. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res. 1985; 29:71–83. PMID: 3981480.
Article93. Robino A, Concas MP, Catamo E, Gasparini P. A brief review of genetic approaches to the study of food preferences: current knowledge and future directions. Nutrients. 2019; 11:1735.
Article94. Breen FM, Plomin R, Wardle J. Heritability of food preferences in young children. Physiol Behav. 2006; 88:443–447. PMID: 16750228.
Article95. Fildes A, van Jaarsveld CH, Llewellyn CH, Fisher A, Cooke L, Wardle J. Nature and nurture in children's food preferences. Am J Clin Nutr. 2014; 99:911–917. PMID: 24477038.
Article96. Keskitalo K, Knaapila A, Kallela M, Palotie A, Wessman M, Sammalisto S, Peltonen L, Tuorila H, Perola M. Sweet taste preferences are partly genetically determined: identification of a trait locus on chromosome 16. Am J Clin Nutr. 2007; 86:55–63. PMID: 17616763.
Article97. Choquette AC, Bouchard L, Drapeau V, Lemieux S, Tremblay A, Bouchard C, Vohl MC, Perusse L. Association between olfactory receptor genes, eating behavior traits and adiposity: results from the Quebec Family Study. Physiol Behav. 2012; 105:772–776. PMID: 22044667.
Article98. Stafford LD, Whittle A. Obese individuals have higher preference and sensitivity to odor of chocolate. Chem Senses. 2015; 40:279–284. PMID: 25771359.
Article99. Dotson CD, Shaw HL, Mitchell BD, Munger SD, Steinle NI. Variation in the gene TAS2R38 is associated with the eating behavior disinhibition in Old Order Amish women. Appetite. 2010; 54:93–99. PMID: 19782709.
Article100. Shigemura N, Shirosaki S, Ohkuri T, Sanematsu K, Islam AA, Ogiwara Y, Kawai M, Yoshida R, Ninomiya Y. Variation in umami perception and in candidate genes for the umami receptor in mice and humans. Am J Clin Nutr. 2009; 90:764S–769S. PMID: 19625681.
Article101. Keller M, Liu X, Wohland T, Rohde K, Gast MT, Stumvoll M, Kovacs P, Tonjes A, Bottcher Y. TAS2R38 and its influence on smoking behavior and glucose homeostasis in the German Sorbs. PLoS One. 2013; 8:e80512. PMID: 24312479.
Article102. Tepper BJ, Koelliker Y, Zhao L, Ullrich NV, Lanzara C, d'Adamo P, Ferrara A, Ulivi S, Esposito L, Gasparini P. Variation in the bitter-taste receptor gene TAS2R38, and adiposity in a genetically isolated population in Southern Italy. Obesity (Silver Spring). 2008; 16:2289–2295. PMID: 18719631.
Article103. Dioszegi J, Llanaj E, Adany R. Genetic background of taste perception, taste preferences, and its nutritional implications: a systematic review. Front Genet. 2019; 10:1272. PMID: 31921309.
Article104. Lipchock SV, Spielman AI, Mennella JA, Mansfield CJ, Hwang LD, Douglas JE, Reed DR. Caffeine bitterness is related to daily caffeine intake and bitter receptor mRNA abundance in human taste tissue. Perception. 2017; 46:245–256. PMID: 28118781.
Article105. Ventura AK, Worobey J. Early influences on the development of food preferences. Curr Biol. 2013; 23:R401–R408. PMID: 23660363.
Article106. Ozdener MH, Subramaniam S, Sundaresan S, Sery O, Hashimoto T, Asakawa Y, Besnard P, Abumrad NA, Khan NA. CD36- and GPR120-mediated Ca2+ signaling in human taste bud cells mediates differential responses to fatty acids and is altered in obese mice. Gastroenterology. 2014; 146:995–1005. PMID: 24412488.107. Thorleifsson G, Walters GB, Gudbjartsson DF, Steinthorsdottir V, Sulem P, Helgadottir A, Styrkarsdottir U, Gretarsdottir S, Thorlacius S, Jonsdottir I, Jonsdottir T, Olafsdottir EJ, Olafsdottir GH, Jonsson T, Jonsson F, Borch-Johnsen K, Hansen T, Andersen G, Jorgensen T, Lauritzen T, Aben KK, Verbeek AL, Roeleveld N, Kampman E, Yanek LR, Becker LC, Tryggvadottir L, Rafnar T, Becker DM, Gulcher J, Kiemeney LA, Pedersen O, Kong A, Thorsteinsdottir U, Stefansson K. Genome-wide association yields new sequence variants at seven loci that associate with measures of obesity. Nat Genet. 2009; 41:18–24. PMID: 19079260.
Article108. Daya M, Pujianto DA, Witjaksono F, Priliani L, Susanto J, Lukito W, Malik SG. Obesity risk and preference for high dietary fat intake are determined by FTO rs9939609 gene polymorphism in selected Indonesian adults. Asia Pac J Clin Nutr. 2019; 28:183–191. PMID: 30896430.109. Merritt DC, Jamnik J, El-Sohemy A. FTO genotype, dietary protein intake, and body weight in a multiethnic population of young adults: a cross-sectional study. Genes Nutr. 2018; 13:4. PMID: 29484031.
Article110. Meng L, Ohman-Gault L, Ma L, Krimm RF. Taste bud-derived BDNF is required to maintain normal amounts of innervation to adult taste buds. eNeuro. 2015; 2:ENEURO.
Article111. McCaffery JM, Jablonski KA, Franks PW, Delahanty LM, Aroda V, Marrero D, Hamman RF, Horton ES, Dagogo-Jack S, Wylie-Rosett J, Barrett-Connor E, Kitabchi A, Knowler WC, Wing RR, Florez JC. Diabetes Prevention Program Research Group. Replication of the association of BDNF and MC4R variants with dietary intake in the diabetes prevention program. Psychosom Med. 2017; 79:224–233. PMID: 27551991.
Article112. Bauer F, Elbers CC, Adan RA, Loos RJ, Onland-Moret NC, Grobbee DE, van Vliet-Ostaptchouk JV, Wijmenga C, van der Schouw YT. Obesity genes identified in genome-wide association studies are associated with adiposity measures and potentially with nutrient-specific food preference. Am J Clin Nutr. 2009; 90:951–959. PMID: 19692490.
Article113. Coffee and Caffeine Genetics Consortium. Cornelis MC, Byrne EM, Esko T, Nalls MA, Ganna A, Paynter N, Monda KL, Amin N, Fischer K, Renstrom F, Ngwa JS, Huikari V, Cavadino A, Nolte IM, Teumer A, Yu K, Marques-Vidal P, Rawal R, Manichaikul A, Wojczynski MK, Vink JM, Zhao JH, Burlutsky G, Lahti J, Mikkila V, Lemaitre RN, Eriksson J, Musani SK, Tanaka T, Geller F, Luan J, Hui J, Magi R, Dimitriou M, Garcia ME, Ho WK, Wright MJ, Rose LM, Magnusson PK, Pedersen NL, Couper D, Oostra BA, Hofman A, Ikram MA, Tiemeier HW, Uitterlinden AG, van Rooij FJ, Barroso I, Johansson I, Xue L, Kaakinen M, Milani L, Power C, Snieder H, Stolk RP, Baumeister SE, Biffar R, Gu F, Bastardot F, Kutalik Z, Jacobs DR Jr, Forouhi NG, Mihailov E, Lind L, Lindgren C, Michaelsson K, Morris A, Jensen M, Khaw KT, Luben RN, Wang JJ, Mannisto S, Perala MM, Kahonen M, Lehtimaki T, Viikari J, Mozaffarian D, Mukamal K, Psaty BM, Doring A, Heath AC, Montgomery GW, Dahmen N, Carithers T, Tucker KL, Ferrucci L, Boyd HA, Melbye M, Treur JL, Mellstrom D, Hottenga JJ, Prokopenko I, Tonjes A, Deloukas P, Kanoni S, Lorentzon M, Houston DK, Liu Y, Danesh J, Rasheed A, Mason MA, Zonderman AB, Franke L, Kristal BS, Karjalainen J, Reed DR, Westra HJ, Evans MK, Saleheen D, Harris TB, Dedoussis G, Curhan G, Stumvoll M, Beilby J, Pasquale LR, Feenstra B, Bandinelli S, Ordovas JM, Chan AT, Peters U, Ohlsson C, Gieger C, Martin NG, Waldenberger M, Siscovick DS, Raitakari O, Eriksson JG, Mitchell P, Hunter DJ, Kraft P, Rimm EB, Boomsma DI, Borecki IB, Loos RJ, Wareham NJ, Vollenweider P, Caporaso N, Grabe HJ, Neuhouser ML, Wolffenbuttel BH, Hu FB, Hypponen E, Jarvelin MR, Cupples LA, Franks PW, Ridker PM, van Duijn CM, Heiss G, Metspalu A, North KE, Ingelsson E, Nettleton JA, van Dam RM, Chasman DI. International Parkinson's Disease Genomics Consortium (IPDGC). North American Brain Expression Consortium (NABEC). UK Brain Expression Consortium (UKBEC). Genome-wide meta-analysis identifies six novel loci associated with habitual coffee consumption. Mol Psychiatry. 2015; 20:647–656. PMID: 25288136.
Article114. van der Klaauw AA, Croizier S, Mendes de Oliveira E, Stadler LKJ, Park S, Kong Y, Banton MC, Tandon P, Hendricks AE, Keogh JM, Riley SE, Papadia S, Henning E, Bounds R, Bochukova EG, Mistry V, O'Rahilly S, Simerly RB, Minchin JEN, Barroso I, Jones EY, Bouret SG, Farooqi IS. INTERVAL. UK10K Consortium. Human semaphorin 3 variants link melanocortin circuit development and energy balance. Cell. 2019; 176:729–742. PMID: 30661757.
Article115. Soberg S, Sandholt CH, Jespersen NZ, Toft U, Madsen AL, von Holstein-Rathlou S, Grevengoed TJ, Christensen KB, Bredie WLP, Potthoff MJ, Solomon TPJ, Scheele C, Linneberg A, Jorgensen T, Pedersen O, Hansen T, Gillum MP, Grarup N. FGF21 is a sugar-induced hormone associated with sweet intake and preference in humans. Cell Metab. 2017; 25:1045–1053. PMID: 28467924.116. von Holstein-Rathlou S, Gillum MP. Fibroblast growth factor 21: an endocrine inhibitor of sugar and alcohol appetite. J Physiol. 2019; 597:3539–3548. PMID: 30921473.
Article117. Frayling TM, Beaumont RN, Jones SE, Yaghootkar H, Tuke MA, Ruth KS, Casanova F, West B, Locke J, Sharp S, Ji Y, Thompson W, Harrison J, Etheridge AS, Gallins PJ, Jima D, Wright F, Zhou Y, Innocenti F, Lindgren CM, Grarup N, Murray A, Freathy RM, Weedon MN, Tyrrell J, Wood AR. A common allele in FGF21 associated with sugar intake is associated with body shape, lower total body-fat percentage, and higher blood pressure. Cell Rep. 2018; 23:327–336. PMID: 29641994.
Article118. Feng P, Jyotaki M, Kim A, Chai J, Simon N, Zhou M, Bachmanov AA, Huang L, Wang H. Regulation of bitter taste responses by tumor necrosis factor. Brain Behav Immun. 2015; 49:32–42. PMID: 25911043.
Article119. Shin YK, Martin B, Kim W, White CM, Ji S, Sun Y, Smith RG, Sevigny J, Tschop MH, Maudsley S, Egan JM. Ghrelin is produced in taste cells and ghrelin receptor null mice show reduced taste responsivity to salty (NaCl) and sour (citric acid) tastants. PLoS One. 2010; 5:e12729. PMID: 20856820.
Article120. Passilly-Degrace P, Chevrot M, Bernard A, Ancel D, Martin C, Besnard P. Is the taste of fat regulated? Biochimie. 2014; 96:3–7. PMID: 23933093.
Article121. Zolotukhin S. Metabolic hormones in saliva: origins and functions. Oral Dis. 2013; 19:219–229. PMID: 22994880.
Article122. Shigemura N, Miura H, Kusakabe Y, Hino A, Ninomiya Y. Expression of leptin receptor (Ob-R) isoforms and signal transducers and activators of transcription (STATs) mRNAs in the mouse taste buds. Arch Histol Cytol. 2003; 66:253–260. PMID: 14527166.
Article123. Nakamura Y, Sanematsu K, Ohta R, Shirosaki S, Koyano K, Nonaka K, Shigemura N, Ninomiya Y. Diurnal variation of human sweet taste recognition thresholds is correlated with plasma leptin levels. Diabetes. 2008; 57:2661–2665. PMID: 18633111.
Article124. Hamann A, Matthaei S. Regulation of energy balance by leptin. Exp Clin Endocrinol Diabetes. 1996; 104:293–300. PMID: 8886745.
Article125. Bartoshuk LM, Duffy VB, Hayes JE, Moskowitz HR, Snyder DJ. Psychophysics of sweet and fat perception in obesity: problems, solutions and new perspectives. Philos Trans R Soc Lond B Biol Sci. 2006; 361:1137–1148. PMID: 16815797.
Article126. Sclafani A. Oral, post-oral and genetic interactions in sweet appetite. Physiol Behav. 2006; 89:525–530. PMID: 16647093.
Article127. Sanematsu K, Nakamura Y, Nomura M, Shigemura N, Ninomiya Y. Diurnal variation of sweet taste recognition thresholds is absent in overweight and obese humans. Nutrients. 2018; 10:297.
Article128. Shigemura N, Ohta R, Kusakabe Y, Miura H, Hino A, Koyano K, Nakashima K, Ninomiya Y. Leptin modulates behavioral responses to sweet substances by influencing peripheral taste structures. Endocrinology. 2004; 145:839–847. PMID: 14592964.
Article129. Jequier E. Leptin signaling, adiposity, and energy balance. Ann N Y Acad Sci. 2002; 967:379–388. PMID: 12079865.130. Herrera Moro Chao D, Argmann C, Van Eijk M, Boot RG, Ottenhoff R, Van Roomen C, Foppen E, Siljee JE, Unmehopa UA, Kalsbeek A, Aerts JM. Impact of obesity on taste receptor expression in extra-oral tissues: emphasis on hypothalamus and brainstem. Sci Rep. 2016; 6:29094. PMID: 27388805.
Article131. Crosson SM, Marques A, Dib P, Dotson CD, Munger SD, Zolotukhin S. Taste receptor cells in mice express receptors for the hormone adiponectin. Chem Senses. 2019; 44:409–422. PMID: 31125082.
Article132. Kim AM, Keenan BT, Jackson N, Chan EL, Staley B, Poptani H, Torigian DA, Pack AI, Schwab RJ. Tongue fat and its relationship to obstructive sleep apnea. Sleep. 2014; 37:1639–1648. PMID: 25197815.
Article133. Clark AA, Dotson CD, Elson AE, Voigt A, Boehm U, Meyerhof W, Steinle NI, Munger SD. TAS2R bitter taste receptors regulate thyroid function. FASEB J. 2015; 29:164–172. PMID: 25342133.
Article134. Martin C, Passilly-Degrace P, Chevrot M, Ancel D, Sparks SM, Drucker DJ, Besnard P. Lipid-mediated release of GLP-1 by mouse taste buds from circumvallate papillae: putative involvement of GPR120 and impact on taste sensitivity. J Lipid Res. 2012; 53:2256–2265. PMID: 22904345.
Article135. Kokrashvili Z, Yee KK, Ilegems E, Iwatsuki K, Li Y, Mosinger B, Margolskee RF. Endocrine taste cells. Br J Nutr. 2014; 111 Suppl 1:S23–S29. PMID: 24382120.
Article136. Kouno T, Akiyama N, Fujieda K, Nanchi I, Okuda T, Iwasaki T, Oka S, Yukioka H. Reduced intake of carbohydrate prevents the development of obesity and impaired glucose metabolism in ghrelin O-acyltransferase knockout mice. Peptides. 2016; 86:145–152. PMID: 27816752.
Article137. De Carli L, Gambino R, Lubrano C, Rosato R, Bongiovanni D, Lanfranco F, Broglio F, Ghigo E, Bo S. Impaired taste sensation in type 2 diabetic patients without chronic complications: a case-control study. J Endocrinol Invest. 2018; 41:765–772. PMID: 29185232.
Article138. Elson AE, Dotson CD, Egan JM, Munger SD. Glucagon signaling modulates sweet taste responsiveness. FASEB J. 2010; 24:3960–3969. PMID: 20547661.
Article139. Doyle ME, Fiori JL, Gonzalez Mariscal I, Liu QR, Goodstein E, Yang H, Shin YK, Santa-Cruz Calvo S, Indig FE, Egan JM. Insulin is transcribed and translated in mammalian taste bud cells. Endocrinology. 2018; 159:3331–3339. PMID: 30060183.
Article140. Baquero AF, Gilbertson TA. Insulin activates epithelial sodium channel (ENaC) via phosphoinositide 3-kinase in mammalian taste receptor cells. Am J Physiol Cell Physiol. 2011; 300:C860–C871. PMID: 21106690.
Article141. Takai S, Watanabe Y, Sanematsu K, Yoshida R, Margolskee RF, Jiang P, Atsuta I, Koyano K, Ninomiya Y, Shigemura N. Effects of insulin signaling on mouse taste cell proliferation. PLoS One. 2019; 14:e0225190. PMID: 31714935.
Article142. Behrens M, Meyerhof W. A role for taste receptors in (neuro)endocrinology? J Neuroendocrinol. 2019; 31:e12691. PMID: 30712315.
Article143. Yoshida R, Noguchi K, Shigemura N, Jyotaki M, Takahashi I, Margolskee RF, Ninomiya Y. Leptin suppresses mouse taste cell responses to sweet compounds. Diabetes. 2015; 64:3751–3762. PMID: 26116698.
Article144. Rodrigues L, Espanca R, Costa AR, Antunes CM, Pomar C, Capela-Silva F, Pinheiro CC, Amado F, Lamy E. Association between salivary leptin levels and taste perception in children. J Nutr Metab. 2017; 2017:7260169. PMID: 28811937.
Article145. Travers SP, Frank ME. Taste bud leptin: sweet dampened at initiation site. Chem Senses. 2015; 40:213–215. PMID: 25740303.
Article146. Katsiougiannis S, Kapsogeorgou EK, Manoussakis MN, Skopouli FN. Salivary gland epithelial cells: a new source of the immunoregulatory hormone adiponectin. Arthritis Rheum. 2006; 54:2295–2299. PMID: 16802369.
Article147. Zhao FL, Shen T, Kaya N, Lu SG, Cao Y, Herness S. Expression, physiological action, and coexpression patterns of neuropeptide Y in rat taste-bud cells. Proc Natl Acad Sci U S A. 2005; 102:11100–11105. PMID: 16040808.
Article148. Haririan H, Andrukhov O, Bottcher M, Pablik E, Wimmer G, Moritz A, Rausch-Fan X. Salivary neuropeptides, stress, and periodontitis. J Periodontol. 2018; 89:9–18. PMID: 28914594.
Article149. La Sala MS, Hurtado MD, Brown AR, Bohorquez DV, Liddle RA, Herzog H, Zolotukhin S, Dotson CD. Modulation of taste responsiveness by the satiation hormone peptide YY. FASEB J. 2013; 27:5022–5033. PMID: 24043261.
Article150. Herness S, Zhao FL. The neuropeptides CCK and NPY and the changing view of cell-to-cell communication in the taste bud. Physiol Behav. 2009; 97:581–591. PMID: 19332083.
Article151. Dotson CD, Geraedts MC, Munger SD. Peptide regulators of peripheral taste function. Semin Cell Dev Biol. 2013; 24:232–239. PMID: 23348523.
Article152. Acosta A, Hurtado MD, Gorbatyuk O, La Sala M, Duncan D, Aslanidi G, Campbell-Thompson M, Zhang L, Herzog H, Voutetakis A, Baum BJ, Zolotukhin S. Salivary PYY: a putative bypass to satiety. PLoS One. 2011; 6:e26137. PMID: 22028819.
Article153. Lu SG, Zhao FL, Herness S. Physiological phenotyping of cholecystokinin-responsive rat taste receptor cells. Neurosci Lett. 2003; 351:157–160. PMID: 14623130.
Article154. Herness S, Zhao FL, Lu SG, Kaya N, Shen T. Expression and physiological actions of cholecystokinin in rat taste receptor cells. J Neurosci. 2002; 22:10018–10029. PMID: 12427859.
Article155. Martin B, Dotson CD, Shin YK, Ji S, Drucker DJ, Maudsley S, Munger SD. Modulation of taste sensitivity by GLP-1 signaling in taste buds. Ann N Y Acad Sci. 2009; 1170:98–101. PMID: 19686117.
Article156. Takai S, Yasumatsu K, Inoue M, Iwata S, Yoshida R, Shigemura N, Yanagawa Y, Drucker DJ, Margolskee RF, Ninomiya Y. Glucagon-like peptide-1 is specifically involved in sweet taste transmission. FASEB J. 2015; 29:2268–2280. PMID: 25678625.
Article157. Ono R. GLP-1 receptor expression in rat major salivary glands and the effects of bilateral maxillary molar extraction on its expression. Kokubyo Gakkai Zasshi. 2015; 81-82:8–14.158. Ohta K, Laborde NJ, Kajiya M, Shin J, Zhu T, Thondukolam AK, Min C, Kamata N, Karimbux NY, Stashenko P, Kawai T. Expression and possible immune-regulatory function of ghrelin in oral epithelium. J Dent Res. 2011; 90:1286–1292. PMID: 21865591.
Article159. Groschl M, Topf HG, Bohlender J, Zenk J, Klussmann S, Dötsch J, Rascher W, Rauh M. Identification of ghrelin in human saliva: production by the salivary glands and potential role in proliferation of oral keratinocytes. Clin Chem. 2005; 51:997–1006. PMID: 15790755.160. Ventre G, Colonna C, Smith J, Alfano D, Moldow R. Salivary VIP concentrations are elevated in humans after acute stress. Peptides. 2013; 49:27–31. PMID: 23994551.
Article161. Sinclair MS, Perea-Martinez I, Abouyared M, St John SJ, Chaudhari N. Oxytocin decreases sweet taste sensitivity in mice. Physiol Behav. 2015; 141:103–110. PMID: 25554481.
Article162. Sinclair MS, Perea-Martinez I, Dvoryanchikov G, Yoshida M, Nishimori K, Roper SD, Chaudhari N. Oxytocin signaling in mouse taste buds. PLoS One. 2010; 5:e11980. PMID: 20700536.
Article163. Martin LE, Nikonova LV, Kay K, Paedae AB, Contreras RJ, Torregrossa AM. Salivary proteins alter taste-guided behaviors and taste nerve signaling in rat. Physiol Behav. 2018; 184:150–161. PMID: 29162505.
Article164. Seta Y, Kataoka S, Toyono T, Toyoshima K. Expression of galanin and the galanin receptor in rat taste buds. Arch Histol Cytol. 2006; 69:273–280. PMID: 17287581.
Article165. Lee RJ, Hariri BM, McMahon DB, Chen B, Doghramji L, Adappa ND, Palmer JN, Kennedy DW, Jiang P, Margolskee RF, Cohen NA. Bacterial d-amino acids suppress sinonasal innate immunity through sweet taste receptors in solitary chemosensory cells. Sci Signal. 2017; 10:eaam7703. PMID: 28874606.
Article166. Wang H, Zhou M, Brand J, Huang L. Inflammation and taste disorders: mechanisms in taste buds. Ann N Y Acad Sci. 2009; 1170:596–603. PMID: 19686199.167. Wang R, van Keeken NM, Siddiqui S, Dijksman LM, Maudsley S, Derval D, van Dam PS, Martin B. Higher TNF-α, IGF-1, and leptin levels are found in tasters than non-tasters. Front Endocrinol (Lausanne). 2014; 5:125. PMID: 25120532.
Article168. Feng P, Zhao H, Chai J, Huang L, Wang H. Expression and secretion of TNF-α in mouse taste buds: a novel function of a specific subset of type II taste cells. PLoS One. 2012; 7:e43140. PMID: 22905218.
Article169. Cohn ZJ, Kim A, Huang L, Brand J, Wang H. Lipopolysaccharide-induced inflammation attenuates taste progenitor cell proliferation and shortens the life span of taste bud cells. BMC Neurosci. 2010; 11:72. PMID: 20537148.
Article170. Zhang J, Zhang L, Zhang S, Yu Q, Xiong F, Huang K, Wang CY, Yang P. HMGB1, an innate alarmin, plays a critical role in chronic inflammation of adipose tissue in obesity. Mol Cell Endocrinol. 2017; 454:103–111. PMID: 28619625.
Article171. Kloting N, Fasshauer M, Dietrich A, Kovacs P, Schon MR, Kern M, Stumvoll M, Bluher M. Insulin-sensitive obesity. Am J Physiol Endocrinol Metab. 2010; 299:E506–E515. PMID: 20570822.172. Bar C, Cohen I, Zhao D, Pothula V, Litskevitch A, Koseki H, Zheng D, Ezhkova E. Polycomb repressive complex 1 controls maintenance of fungiform papillae by repressing sonic hedgehog expression. Cell Rep. 2019; 28:257–266. PMID: 31269445.
Article173. Wang C, Shan S, Wang C, Wang J, Li J, Hu G, Dai K, Li Q, Zhang X. Mechanical stimulation promote the osteogenic differentiation of bone marrow stromal cells through epigenetic regulation of Sonic Hedgehog. Exp Cell Res. 2017; 352:346–356. PMID: 28215635.
Article174. Zhou Q, Guan W, Qiao H, Cheng Y, Li Z, Zhai X, Zhou Y. GATA binding protein 2 mediates leptin inhibition of PPARγ1 expression in hepatic stellate cells and contributes to hepatic stellate cell activation. Biochim Biophys Acta. 2014; 1842:2367–2377. PMID: 25305367.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Iatrogenic Taste Disorder
- Immunohistochemical study on the dopaminergic and norepinephrinergic taste cells in rat taste buds
- Immuno-electronmicroscopic study on the serotoninergic taste cells and calcitonin gene-related peptide nerve fibers in mouse taste buds
- The Role of the Sweet Taste Receptor in Enteroendocrine Cells and Pancreatic beta-Cells
- Expression of Neurotrophic Factors and Their Receptors in Rat Posterior Taste Bud Cells