Korean J Physiol Pharmacol.
2021 Mar;25(2):159-166. 10.4196/kjpp.2021.25.2.159.
NOX4/Src regulates ANP secretion through activating ERK1/2 and Akt/GATA4 signaling in beating rat hypoxic atria
- Affiliations
-
- 1Department of Physiology, School of Medicine, Yanbian University, Yanji 133-002, China
- 2Institute of Clinical Medicine, Yanbian University, Yanji 133-002, China
- 3Inner Mongolia University for Nationalities, Tongliao 028000, China
- 4Cellular Function Research Center, Yanbian University, Yanji 133-002, China
- KMID: 2512959
- DOI: http://doi.org/10.4196/kjpp.2021.25.2.159
Abstract
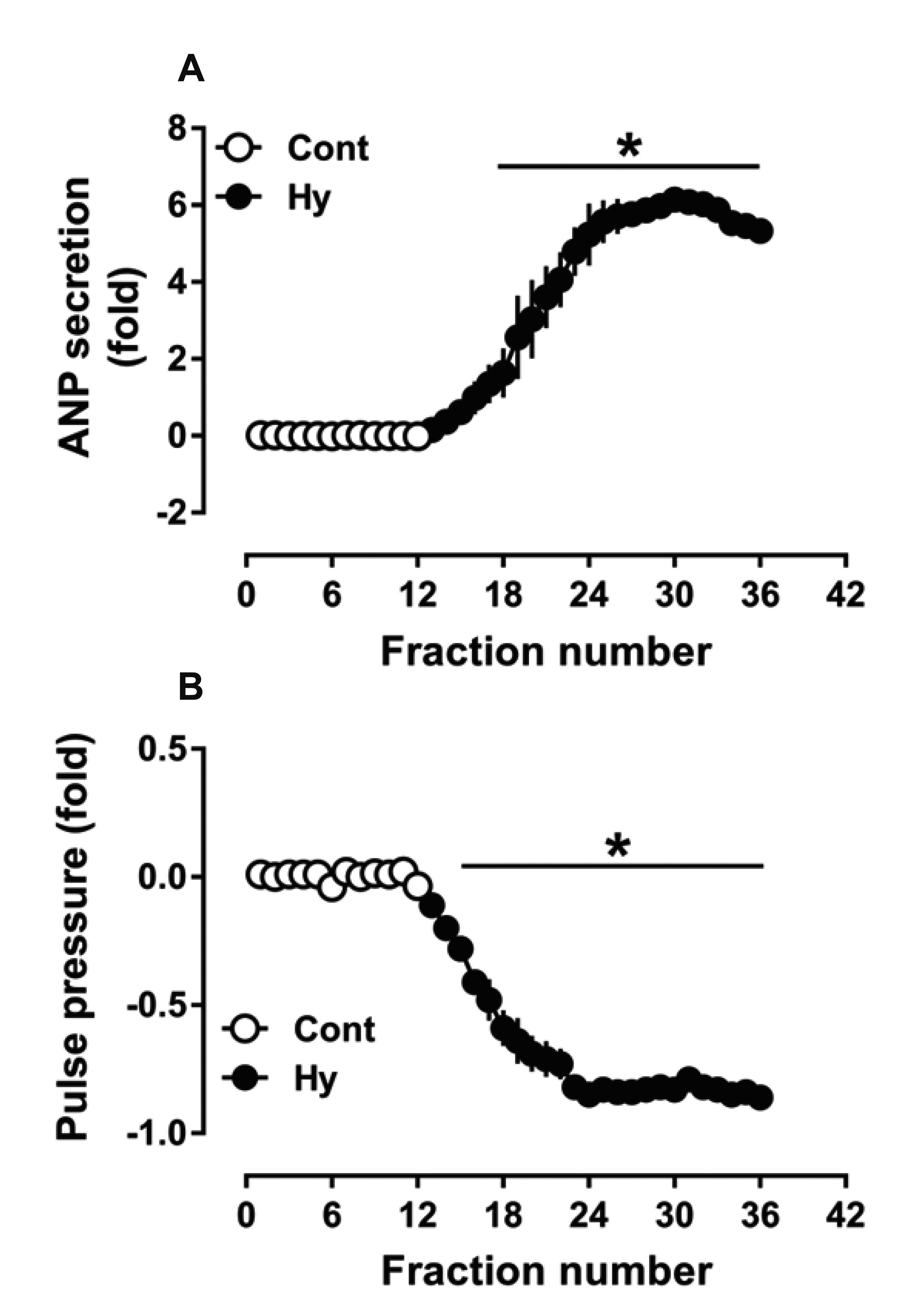
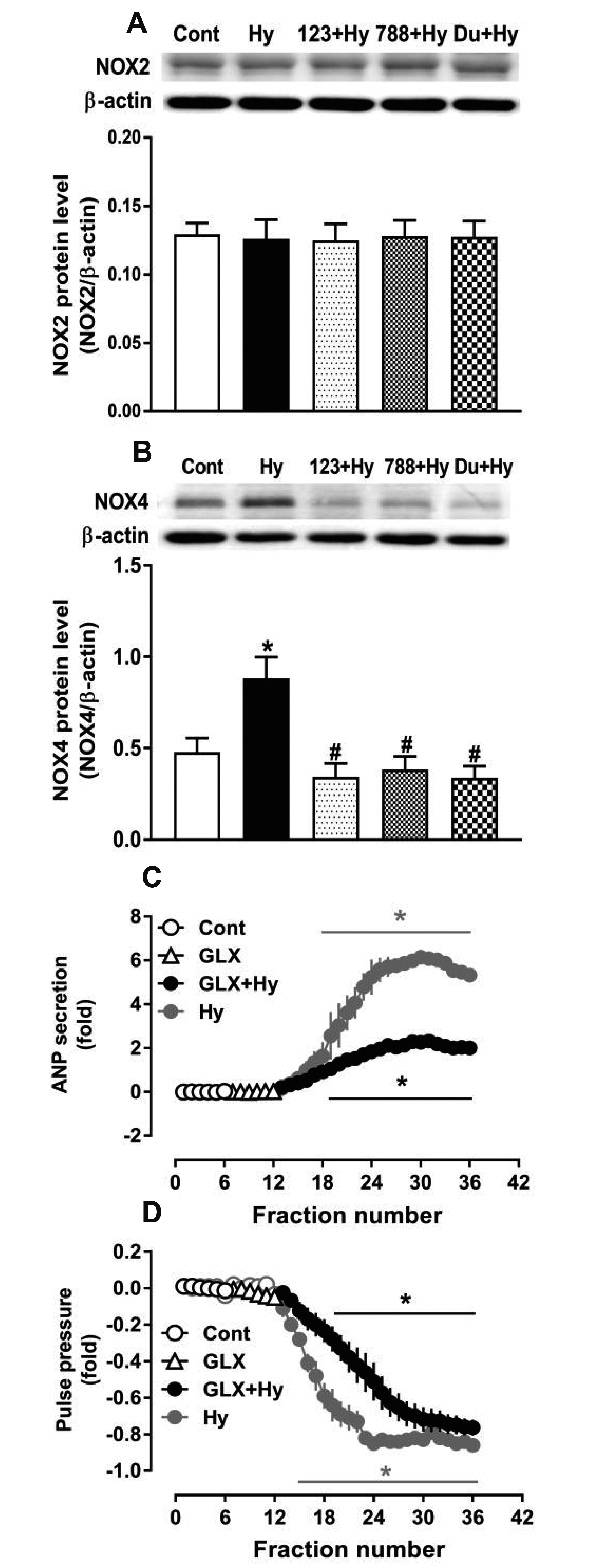
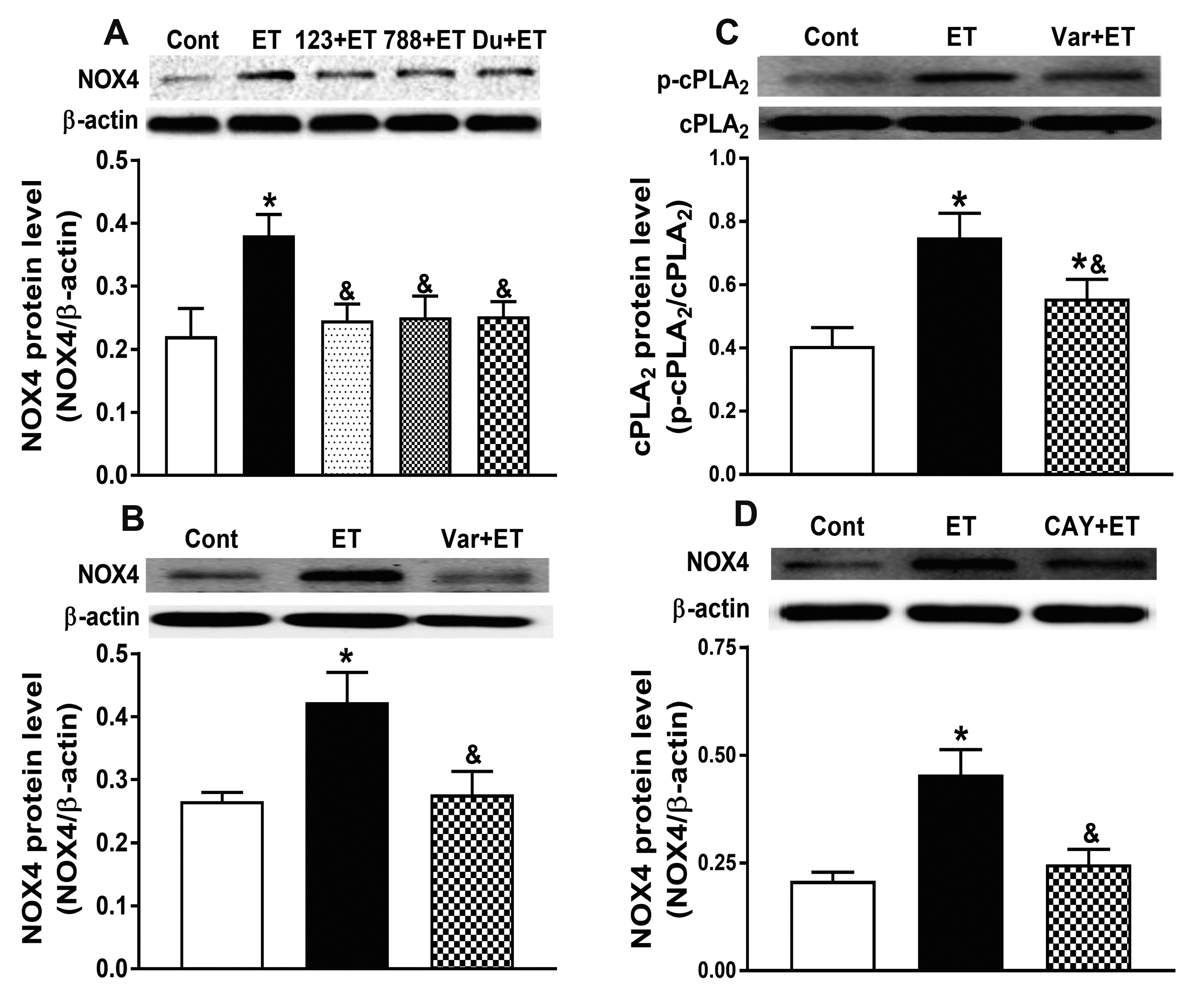
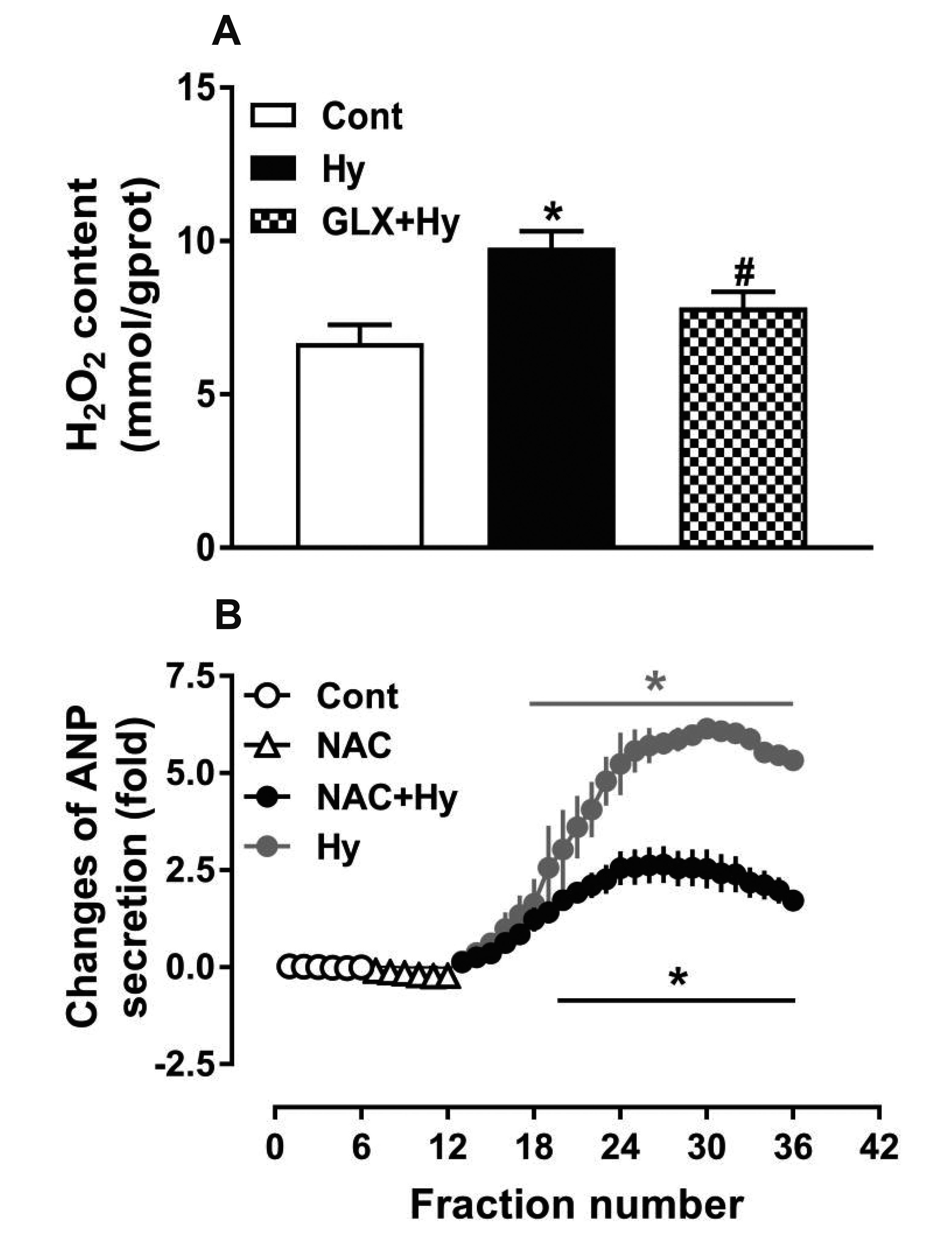
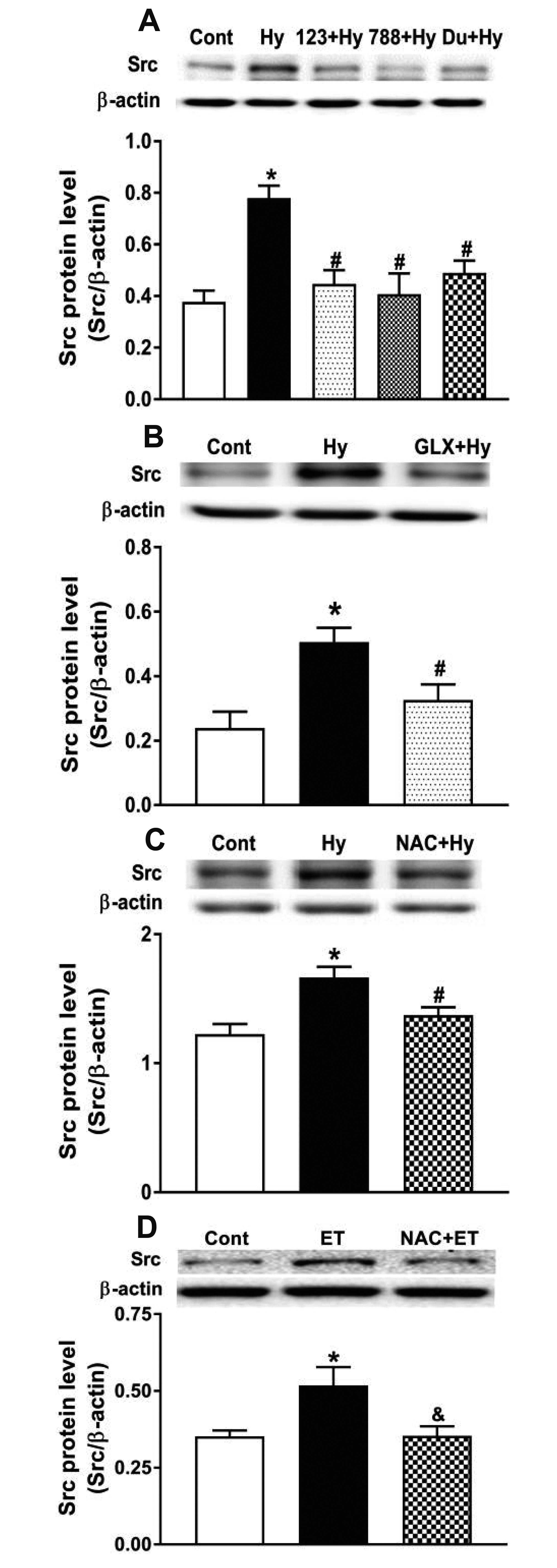
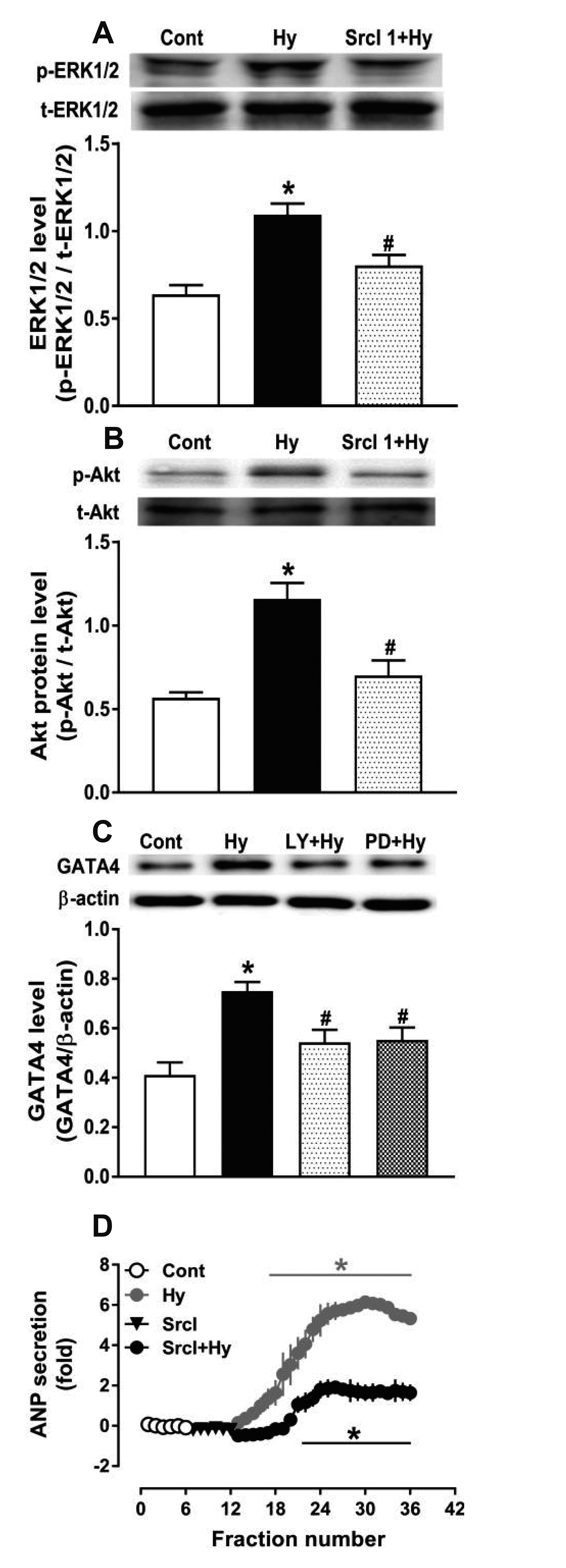
- Nicotinamide adenine dinucleotide phosphate oxidases (NOXs) are the major enzymatic source of reactive oxygen species (ROS). NOX2 and NOX4 are expressed in the heart but its role in hypoxia-induced atrial natriuretic peptide (ANP) secretion is unclear. This study investigated the effect of NOX on ANP secretion induced by hypoxia in isolated beating rat atria. The results showed that hypoxia significantly upregulated NOX4 but not NOX2 expression, which was completely abolished by endothelin-1 (ET-1) type A and B receptor antagonists BQ123 (0.3 µM) and BQ788 (0.3 µM). ET-1-upregulated NOX4 expression was also blocked by antagonists of secreted phospholipase A2 (sPLA2; varespladib, 5.0 µM) and cytosolic PLA2 (cPLA2; CAY10650, 120.0 nM), and ET-1-induced cPLA2 expression was inhibited by varespladib under normoxia. Moreover, hypoxia-increased ANP secretion was evidently attenuated by the NOX4 antagonist GLX351322 (35.0 µM) and inhibitor of ROS N-Acetyl-D-cysteine (NAC, 15.0 mM), and hypoxia-increased production of ROS was blocked by GLX351322. In addition, hypoxia markedly upregulated Src expression, which was blocked by ET receptors, NOX4, and ROS antagonists. ET-1-increased Src expression was also inhibited by NAC under normoxia. Furthermore, hypoxiaactivated extracellular signal-regulated kinase 1/2 (ERK1/2) and protein kinase B (Akt) were completely abolished by Src inhibitor 1 (1.0 µM), and hypoxia-increased GATA4 was inhibited by the ERK1/2 and Akt antagonists PD98059 (10.0 µM) and LY294002 (10.0 µM), respectively. However, hypoxia-induced ANP secretion was substantially inhibited by Src inhibitor. These results indicate that NOX4/Src modulated by ET-1 regulates ANP secretion by activating ERK1/2 and Akt/GATA4 signaling in isolated beating rat hypoxic atria.
Figure
Reference
-
1. Zhang Y, Murugesan P, Huang K, Cai H. 2020; NADPH oxidases and oxidase crosstalk in cardiovascular diseases: novel therapeutic targets. Nat Rev Cardiol. 17:170–194. DOI: 10.1038/s41569-019-0260-8. PMID: 31591535. PMCID: PMC7880919.
Article2. Zhang M, Perino A, Ghigo A, Hirsch E, Shah AM. 2013; NADPH oxidases in heart failure: poachers or gamekeepers? Antioxid Redox Signal. 18:1024–1041. DOI: 10.1089/ars.2012.4550. PMID: 22747566. PMCID: PMC3567780.
Article3. Bedard K, Krause KH. 2007; The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 87:245–313. DOI: 10.1152/physrev.00044.2005. PMID: 17237347.
Article4. Bendall JK, Cave AC, Heymes C, Gall N, Shah AM. 2002; Pivotal role of a gp91(phox)-containing NADPH oxidase in angiotensin II-induced cardiac hypertrophy in mice. Circulation. 105:293–296. DOI: 10.1161/hc0302.103712. PMID: 11804982.5. Rodiño-Janeiro BK, Paradela-Dobarro B, Castiñeiras-Landeira MI, Raposeiras-Roubín S, González-Juanatey JR, Alvarez E. 2013; Current status of NADPH oxidase research in cardiovascular pharmacology. Vasc Health Risk Manag. 9:401–428. DOI: 10.2147/VHRM.S33053. PMID: 23983473. PMCID: PMC3750863.6. Cho KW, Seul KH, Ryu H, Kim SH, Koh GY. 1988; Characteristics of distension-induced release of immunoreactive atrial natriuretic peptide in isolated perfused rabbit atria. Regul Pept. 22:333–345. DOI: 10.1016/0167-0115(88)90110-3. PMID: 2973090.
Article7. McGrath MF, de Bold ML, de Bold AJ. 2005; The endocrine function of the heart. Trends Endocrinol Metab. 16:469–477. DOI: 10.1016/j.tem.2005.10.007. PMID: 16269246.
Article8. De Vito P, Incerpi S, Pedersen JZ, Luly P. 2010; Atrial natriuretic peptide and oxidative stress. Peptides. 31:1412–1419. DOI: 10.1016/j.peptides.2010.04.001. PMID: 20385186.
Article9. Arjamaa O, Nikinmaa M. 2011; Hypoxia regulates the natriuretic peptide system. Int J Physiol Pathophysiol Pharmacol. 3:191–201. DOI: 10.1016/j.ijcard.2011.11.055. PMID: 22188987. PMCID: PMC3175745.10. Wang D, Gladysheva IP, Fan TH, Sullivan R, Houng AK, Reed GL. 2014; Atrial natriuretic peptide affects cardiac remodeling, function, heart failure, and survival in a mouse model of dilated cardiomyopathy. Hypertension. 63:514–519. DOI: 10.1161/HYPERTENSIONAHA.113.02164. PMID: 24379183. PMCID: PMC4015109.
Article11. Hong L, Xi J, Zhang Y, Tian W, Xu J, Cui X, Xu Z. 2012; Atrial natriuretic peptide prevents the mitochondrial permeability transition pore opening by inactivating glycogen synthase kinase 3β via PKG and PI3K in cardiac H9c2 cells. Eur J Pharmacol. 695:13–19. DOI: 10.1016/j.ejphar.2012.07.053. PMID: 22975711.
Article12. MacKay CE, Knock GA. 2015; Control of vascular smooth muscle function by Src-family kinases and reactive oxygen species in health and disease. J Physiol. 593:3815–3828. DOI: 10.1113/jphysiol.2014.285304. PMID: 25384773. PMCID: PMC4575571.
Article13. Li X, Han ZN, Liu Y, Hong L, Cui BR, Cui X. 2019; Endogenous ET-1 promotes ANP secretion through activation of COX2-L-PGDS-PPARγ signaling in hypoxic beating rat atria. Peptides. 122:170150. DOI: 10.1016/j.peptides.2019.170150. PMID: 31541683.
Article14. Kuroda J, Ago T, Matsushima S, Zhai P, Schneider MD, Sadoshima J. 2010; NADPH oxidase 4 (Nox4) is a major source of oxidative stress in the failing heart. Proc Natl Acad Sci U S A. 107:15565–15570. DOI: 10.1073/pnas.1002178107. PMID: 20713697. PMCID: PMC2932625.
Article15. Zhang M, Brewer AC, Schröder K, Santos CX, Grieve DJ, Wang M, Anilkumar N, Yu B, Dong X, Walker SJ, Brandes RP, Shah AM. 2010; NADPH oxidase-4 mediates protection against chronic load-induced stress in mouse hearts by enhancing angiogenesis. Proc Natl Acad Sci U S A. 107:18121–18126. DOI: 10.1073/pnas.1009700107. PMID: 20921387. PMCID: PMC2964252.
Article16. Ruetten H, Thiemermann C. 1997; Endothelin-1 stimulates the biosynthesis of tumour necrosis factor in macrophages: ET-receptors, signal transduction and inhibition by dexamethasone. J Physiol Pharmacol. 48:675–688. PMID: 9444616.17. De Windt LJ, Willemsen PH, Pöpping S, Van der Vusse GJ, Reneman RS, Van Bilsen M. 1997; Cloning and cellular distribution of a group II phospholipase A2 expressed in the heart. J Mol Cell Cardiol. 29:2095–2106. DOI: 10.1006/jmcc.1997.0444. PMID: 9281442.18. Su J, An XR, Li Q, Li XX, Cong XD, Xu M. 2018; Improvement of vascular dysfunction by argirein through inhibiting endothelial cell apoptosis associated with ET-1/Nox4 signal pathway in diabetic rats. Sci Rep. 8:12620. DOI: 10.1038/s41598-018-30386-w. PMID: 30135489. PMCID: PMC6105644.
Article19. Tam SW, Feng R, Lau WK, Law AC, Yeung PK, Chung SK. 2019; Endothelin type B receptor promotes cofilin rod formation and dendritic loss in neurons by inducing oxidative stress and cofilin activation. J Biol Chem. 294:12495–12506. DOI: 10.1074/jbc.RA118.005155. PMID: 31248984. PMCID: PMC6699845.
Article20. Gao S, Yuan K, Shah A, Kim JS, Park WH, Kim SH. 2011; Suppression of high pacing-induced ANP secretion by antioxidants in isolated rat atria. Peptides. 32:2467–2473. DOI: 10.1016/j.peptides.2011.10.022. PMID: 22063193.
Article21. Roskoski R Jr. 2015; Src protein-tyrosine kinase structure, mechanism, and small molecule inhibitors. Pharmacol Res. 94:9–25. DOI: 10.1016/j.phrs.2015.01.003. PMID: 25662515.
Article22. Yuan Z, McCauley R, Chen-Scarabelli C, Abounit K, Stephanou A, Barry SP, Knight R, Saravolatz SF, Saravolatz LD, Ulgen BO, Scarabelli GM, Faggian G, Mazzucco A, Saravolatz L, Scarabelli TM. 2010; Activation of Src protein tyrosine kinase plays an essential role in urocortin-mediated cardioprotection. Mol Cell Endocrinol. 325:1–7. DOI: 10.1016/j.mce.2010.04.013. PMID: 20416357.
Article23. Zhang QL, Cui BR, Li HY, Li P, Hong L, Liu LP, Ding DZ, Cui X. 2013; MAPK and PI3K pathways regulate hypoxia-induced atrial natriuretic peptide secretion by controlling HIF-1 alpha expression in beating rabbit atria. Biochem Biophys Res Commun. 438:507–512. DOI: 10.1016/j.bbrc.2013.07.106. PMID: 23916614.
Article24. Hayek S, Nemer M. 2011; Cardiac natriuretic peptides: from basic discovery to clinical practice. Cardiovasc Ther. 29:362–376. DOI: 10.1111/j.1755-5922.2010.00152.x. PMID: 20433683.
Article25. Temsah R, Nemer M. 2005; GATA factors and transcriptional regulation of cardiac natriuretic peptide genes. Regul Pept. 128:177–185. DOI: 10.1016/j.regpep.2004.12.026. PMID: 15837526.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The WNT/Ca2+ pathway promotes atrial natriuretic peptide secretion by activating protein kinase C/transforming growth factor-β activated kinase 1/activating transcription factor 2 signaling in isolated beating rat atria
- Peroxisome proliferator-activated receptor γ is essential for secretion of ANP induced by prostaglandin D₂ in the beating rat atrium
- Characteristics of hypoxia-induced ANP Secretion in Perfused Beating Atria
- Carbon monoxide releasing molecule-2 suppresses stretchactivated atrial natriuretic peptide secretion by activating largeconductance calcium-activated potassium channels
- Comparative effects of angiotensin II and angiotensin-(4-8) on blood pressure and ANP secretion in rats