Ann Lab Med.
2021 Jul;41(4):419-423. 10.3343/alm.2021.41.4.419.
Concordance of Three Automated Procalcitonin Immunoassays at Medical Decision Points
- Affiliations
-
- 1Department of Laboratory Medicine, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea
- 2Department of Laboratory Medicine Research Institute of Bacterial Resistance, Yonsei University College of Medicine, Seoul, Korea
- KMID: 2512723
- DOI: http://doi.org/10.3343/alm.2021.41.4.419
Abstract
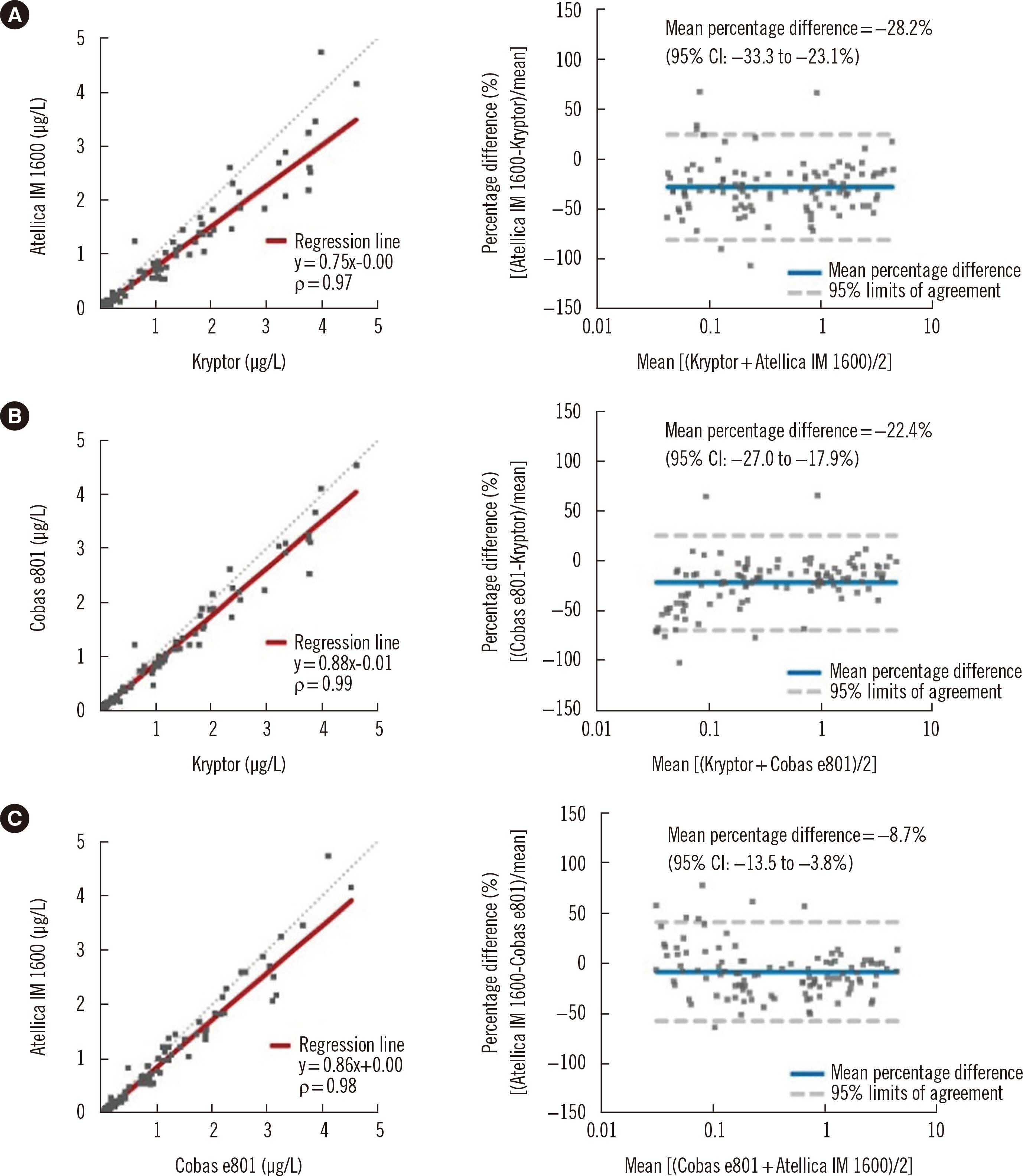
- Procalcitonin (PCT) is a useful bacterial infection biomarker with the potential for guiding antibiotic therapy. We evaluated the concordance of three automated PCT immunoassays: Kryptor (BRAHMS GmbH, Hennigsdorf, Germany), Atellica IM 1600 (Siemens Healthcare Diagnostics, Munich, Germany), and Cobas e801 (Roche Diagnostics, Mannheim, Germany). In 119 serum samples with a PCT concentration < 5.00 μg/L, Kryptor (reference assay) was compared with the other two immunoassays by Spearman’s rank correlation, regression analysis, and concordance at two antibiotic stewardship medical decision points: 0.25 and 0.50 μg/L. The Atellica IM 1600 and Cobas e801 results showed high correlations with those of Kryptor, with correlation coefficient (ρ) values of 0.97 and 0.99, respectively. However, negative biases were observed in both immunoassays (slope/y-intercept: 0.75/–0.00 for Atellica IM 1600; 0.88/–0.01 for Cobas e801). Atellica IM 1600 and Cobas e801 demonstrated excellent concordance with Kryptor at both medical decision points, with linearly weighted κ values of 0.90 and 0.92, respectively, despite discrepancies, which were more prominent at the 0.25 μg/L medical decision point. Based on these biases and discrepancies, the alternate use of different PCT immunoassays in repeat examinations is inadvisable. Standardization is required before comparing the results of different PCT immunoassays.
Keyword
Figure
Reference
-
1. Samsudin I, Vasikaran SD. 2017; Clinical utility and measurement of procalcitonin. Clin Biochem Rev. 38:59–68.2. Schuetz P, Beishuizen A, Broyles M, Ferrer R, Gavazzi G, Gluck EH, et al. 2019; Procalcitonin (PCT)-guided antibiotic stewardship: an international experts consensus on optimized clinical use. Clin Chem Lab Med. 57:1308–18. DOI: 10.1515/cclm-2018-1181. PMID: 30721141.
Article3. Meisner M. 2014; Update on procalcitonin measurements. Ann Lab Med. 34:263–73. DOI: 10.3343/alm.2014.34.4.263. PMID: 24982830. PMCID: PMC4071182.
Article4. Bouadma L, Luyt CE, Tubach F, Cracco C, Alvarez A, Schwebel C, et al. 2010; Use of procalcitonin to reduce patients' exposure to antibiotics in intensive care units (PRORATA trial): a multicentre randomised controlled trial. Lancet. 375:463–74. DOI: 10.1016/S0140-6736(09)61879-1.
Article5. Schuetz P, Wirz Y, Sager R, Christ-Crain M, Stolz D, Tamm M, et al. 2018; Effect of procalcitonin-guided antibiotic treatment on mortality in acute respiratory infections: a patient level meta-analysis. Lancet Infect Dis. 18:95–107. DOI: 10.1016/S1473-3099(17)30592-3.6. Christ-Crain M, Jaccard-Stolz D, Bingisser R, Gencay MM, Huber PR, Tamm M, et al. 2004; Effect of procalcitonin-guided treatment on antibiotic use and outcome in lower respiratory tract infections: cluster-randomised, single-blinded intervention trial. Lancet. 363:600–7. DOI: 10.1016/S0140-6736(04)15591-8.
Article7. Heilmann E, Gregoriano C, Wirz Y, Luyt CE, Wolff M, Chastre J, et al. 2020; Association of kidney function with effectiveness of procalcitonin-guided antibiotic treatment: a patient-level meta-analysis from randomized controlled trials. Clin Chem Lab Med. doi:10.1515/cclm-2020-0931. DOI: 10.1515/cclm-2020-0931. PMID: 32986609.8. Schuetz P, Bretscher C, Bernasconi L, Mueller B. 2017; Overview of procalcitonin assays and procalcitonin-guided protocols for the management of patients with infections and sepsis. Expert Rev Mol Diagn. 17:593–601. DOI: 10.1080/14737159.2017.1324299. PMID: 28443360.
Article9. U.S. Food & Drug Administration. Medical device databases: CLIA-clinical laboratory improvement amendments. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfCLIA/search.cfm. Updated on Dec 2020.10. Schuetz P, Birkhahn R, Sherwin R, Jones AE, Singer A, Kline JA, et al. 2017; Serial procalcitonin predicts mortality in severe sepsis patients: results from the multicenter procalcitonin monitoring sepsis (MOSES) study. Crit Care Med. 45:781–9. DOI: 10.1097/CCM.0000000000002321. PMID: 28257335. PMCID: PMC5389588.11. de Wolf HK, Gunnewiek JK, Berk Y, van den Ouweland J, de Metz M. 2009; Comparison of a new procalcitonin assay from Roche with the established method on the Brahms Kryptor. Clin Chem. 55:1043–4. DOI: 10.1373/clinchem.2008.117655. PMID: 19264851.
Article12. Hausfater P, Brochet C, Freund Y, Charles V, Bernard M. 2010; Procalcitonin measurement in routine emergency medicine practice: comparison between two immunoassays. Clin Chem Lab Med. 48:501–4. DOI: 10.1515/CCLM.2010.091. PMID: 20148728.
Article13. Dipalo M, Guido L, Micca G, Pittalis S, Locatelli M, Motta A, et al. 2015; Multicenter comparison of automated procalcitonin immunoassays. Pract Lab Med. 2:22–8. DOI: 10.1016/j.plabm.2015.07.001. PMID: 28932801. PMCID: PMC5597721.
Article14. Kutz A, Hausfater P, Oppert M, Alan M, Grolimund E, Gast C, et al. 2016; Comparison between B·R·A·H·M·S PCT direct, a new sensitive point-of-care testing device for rapid quantification of procalcitonin in emergency department patients and established reference methods-a prospective multinational trial. Clin Chem Lab Med. 54:577–84. DOI: 10.1515/cclm-2015-0437. PMID: 26426890.
Article15. Ceriotti F, Marino I, Motta A, Carobene A. 2017; Analytical evaluation of the performances of Diazyme and BRAHMS procalcitonin applied to Roche Cobas in comparison with BRAHMS PCT-sensitive Kryptor. Clin Chem Lab Med. 56:162–9. DOI: 10.1515/cclm-2017-0159. PMID: 28809746.
Article16. Soh A, Binder L, Clough M, Hernandez MH, Lefèvre G, Mostert K, et al. 2018; Comparison of the novel ARCHITECT procalcitonin assay with established procalcitonin assay systems. Pract Lab Med. 12:e00110. DOI: 10.1016/j.plabm.2018.e00110. PMID: 30519621. PMCID: PMC6249413.
Article17. Chambliss AB, Hayden J, Colby JM. 2019; Evaluation of procalcitonin immunoassay concordance near clinical decision points. Clin Chem Lab Med. 57:1414–21. DOI: 10.1515/cclm-2018-1362. PMID: 30763263.
Article18. Gruzdys V, Cahoon K, Pearson L, Lehman CM. 2019; Method verification shows a negative bias between 2 procalcitonin methods at medical decision concentrations. J Appl Lab Med. 4:69–77. DOI: 10.1373/jalm.2018.028449. PMID: 31639709.
Article19. Lippi G, Salvagno GL, Gelati M, Pucci M, Lo Cascio C, Demonte D, et al. 2019; Two-center comparison of 10 fully-automated commercial procalcitonin (PCT) immunoassays. Clin Chem Lab Med. 58:77–84. DOI: 10.1515/cclm-2019-0888. PMID: 31539351.
Article20. Lippi G, Salvagno GL, Gelati M, Pucci M, Demonte D, Faggian D, et al. 2020; Analytical evaluation of the new Beckman Coulter Access procalcitonin (PCT) chemiluminescent immunoassay. Diagnostics (Basel). 10:128. DOI: 10.3390/diagnostics10030128. PMID: 32111028. PMCID: PMC7151122.
Article21. Eidizadeh A, Asif AR, von Ahsen N, Binder L, Schnelle M. 2019; Differences in procalcitonin measurements between three BRAHMS-partnered immunoassays (Liaison, Elecsys and Architect). Clin Chem Lab Med. 57:e207–10. DOI: 10.1515/cclm-2018-0916. PMID: 30325730.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of 3 Automated Immunoassays for Hepatitis B Surface Antigen
- Comparison of Three Automated Calcitonin Immunoassays for Evaluating the Equivalence Near the Clinical Decision Point
- Comparison of Three Assay Systems for Qualitative and Quantitative Results of Hepatitis B Surface Antibody
- Evaluation of the Automated Cross-Matching Instrument, ORTHO VISION, for Use in Blood Banks
- Comparison of three types of analyzers for urine protein-tocreatinine ratios in dogs