J Korean Med Sci.
2021 Feb;36(6):e50. 10.3346/jkms.2021.36.e50.
Motor Asymmetry and Interocular Retinal Thickness in Parkinson's Disease
- Affiliations
-
- 1Department of Ophthalmology, Hallym University Sacred Heart Hospital, Hallym University College of Medicine, Anyang, Korea
- 2Department of Neurology, Hallym University Dongtan Sacred Heart Hospital, Hallym University College of Medicine, Hwaseong, Korea
- 3Department of Ophthalmology, Hallym University Dongtan Sacred Heart Hospital, Hallym University College of Medicine, Hwaseong, Korea
- KMID: 2512632
- DOI: http://doi.org/10.3346/jkms.2021.36.e50
Abstract
- Background
To analyze the relationship between interocular difference of retinal thickness and motor asymmetry in Parkinson's disease (PD).
Methods
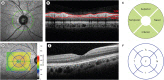
Prospective case-control series analyzed 62 eyes of 31 patients with PD and 62 eyes of 31 age- and sex-matched control. Ophthalmologic examinations including optical coherence tomography (OCT) scans were performed in both groups, and in the patients with PD, motor function was evaluated on the Unified Parkinson's Disease Rating Scale part III (UPDRS-III) to determine the clinically more affected side. Peripapillary retinal nerve fiber layer thickness (pRNFLT) and macular retinal thickness (mRT) were measured in both eyes, after which the interocular asymmetry of the OCT parameters was determined. Additionally, the more and less affected sides of the UPDRS-III were evaluated using Symmetric index.
Results
The average and quadrant pRNFLT and mRT values between the two groups were not different, but the interocular asymmetry of the average mRT and asymmetry index of retinal thickness (AIRT) of temporal mRT were significantly higher in the PD patients than in the controls (P = 0.026 and 0.044). The sum of UPDRS-III showed a discrepancy between the more and less affected sides (P = 0.002); the calculated Symmetric index was 0.21 ± 0.19, which suggested asymmetric motor symptoms. The Symmetric index of UPDRS-III showed significant relations for interocular asymmetry of superior mRT and AIRT of average mRT (P = 0.001 and 0.008).
Conclusion
In the PD patients, the interocular asymmetry of mRT was larger than in the controls, and the motor symptoms were asymmetric. Additionally, the interocular asymmetry of mRT showed a significant correlation with motor-symptom laterality.
Figure
Reference
-
1. Birch J, Kolle RU, Kunkel M, Paulus W, Upadhyay P. Acquired colour deficiency in patients with Parkinson's disease. Vision Res. 1998; 38(21):3421–3426. PMID: 9893859.
Article2. Matsui H, Udaka F, Tamura A, Oda M, Kubori T, Nishinaka K, et al. Impaired visual acuity as a risk factor for visual hallucinations in Parkinson's disease. J Geriatr Psychiatry Neurol. 2006; 19(1):36–40. PMID: 16449759.
Article3. Djamgoz MB, Hankins MW, Hirano J, Archer SN. Neurobiology of retinal dopamine in relation to degenerative states of the tissue. Vision Res. 1997; 37(24):3509–3529. PMID: 9425527.
Article4. Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W, et al. Optical coherence tomography. Science. 1991; 254(5035):1178–1181. PMID: 1957169.
Article5. Inzelberg R, Ramirez JA, Nisipeanu P, Ophir A. Retinal nerve fiber layer thinning in Parkinson disease. Vision Res. 2004; 44(24):2793–2797. PMID: 15342223.
Article6. Aaker GD, Myung JS, Ehrlich JR, Mohammed M, Henchcliffe C, Kiss S. Detection of retinal changes in Parkinson's disease with spectral-domain optical coherence tomography. Clin Ophthalmol. 2010; 4:1427–1432. PMID: 21188154.7. Altintaş O, Işeri P, Özkan B, Cağlar Y. Correlation between retinal morphological and functional findings and clinical severity in Parkinson's disease. Doc Ophthalmol. 2008; 116(2):137–146. PMID: 17962989.
Article8. Albrecht P, Müller AK, Südmeyer M, Ferrea S, Ringelstein M, Cohn E, et al. Optical coherence tomography in parkinsonian syndromes. PLoS One. 2012; 7(4):e34891. PMID: 22514688.
Article9. Roth NM, Saidha S, Zimmermann H, Brandt AU, Isensee J, Benkhellouf-Rutkowska A, et al. Photoreceptor layer thinning in idiopathic Parkinson's disease. Mov Disord. 2014; 29(9):1163–1170. PMID: 24789530.
Article10. Tsironi EE, Dastiridou A, Katsanos A, Dardiotis E, Veliki S, Patramani G, et al. Perimetric and retinal nerve fiber layer findings in patients with Parkinson's disease. BMC Ophthalmol. 2012; 12(1):54. PMID: 23031247.
Article11. Hoehn MM, Yahr MD. Parkinsonism: onset, progression, and mortality. 1967. Neurology. 1998; 50(2):318. PMID: 9484345.12. Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992; 55(3):181–184. PMID: 1564476.
Article13. Martinu K, Nagano-Saito A, Fogel S, Monchi O. Asymmetrical effect of levodopa on the neural activity of motor regions in PD. PLoS One. 2014; 9(11):e111600. PMID: 25369523.
Article14. Tanner JJ, Levy SA, Schwab NA, Hizel LP, Nguyen PT, Okun MS, et al. Marked brain asymmetry with intact cognitive functioning in idiopathic Parkinson's disease: a longitudinal analysis. Clin Neuropsychol. 2017; 31(3):654–675. PMID: 27813459.
Article15. Marsden CD, Parkes JD, Quinn N. 7 - Fluctuations of disability in Parkinson's disease – clinical aspects. Mov Disord. 1981; 96–122.16. Altemir I, Oros D, Elía N, Polo V, Larrosa JM, Pueyo V. Retinal asymmetry in children measured with optical coherence tomography. Am J Ophthalmol. 2013; 156(6):1238–1243.e1. PMID: 24075424.
Article17. Yang M, Wang W, Xu Q, Tan S, Wei S. Interocular symmetry of the peripapillary choroidal thickness and retinal nerve fibre layer thickness in healthy adults with isometropia. BMC Ophthalmol. 2016; 16(1):182. PMID: 27756260.
Article18. Lin PW, Chang HW, Lai IC, Tsai JC, Poon YC. Intraocular retinal thickness asymmetry in early stage of primary open angle glaucoma and normal tension glaucoma. Int J Ophthalmol. 2018; 11(8):1342–1351. PMID: 30140639.
Article19. Shreve LA, Velisar A, Malekmohammadi M, Koop MM, Trager M, Quinn EJ, et al. Subthalamic oscillations and phase amplitude coupling are greater in the more affected hemisphere in Parkinson's disease. Clin Neurophysiol. 2017; 128(1):128–137. PMID: 27889627.
Article20. Plotnik M, Giladi N, Balash Y, Peretz C, Hausdorff JM. Is freezing of gait in Parkinson's disease related to asymmetric motor function? Ann Neurol. 2005; 57(5):656–663. PMID: 15852404.
Article21. Uitti RJ, Baba Y, Whaley NR, Wszolek ZK, Putzke JD. Parkinson disease: handedness predicts asymmetry. Neurology. 2005; 64(11):1925–1930. PMID: 15955945.
Article22. Ham JH, Lee JJ, Kim JS, Lee PH, Sohn YH. Is dominant-side onset associated with a better motor compensation in Parkinson's disease? Mov Disord. 2015; 30(14):1921–1925. PMID: 26408124.
Article23. Huang P, Tan YY, Liu DQ, Herzallah MM, Lapidow E, Wang Y, et al. Motor-symptom laterality affects acquisition in Parkinson's disease: a cognitive and functional magnetic resonance imaging study. Mov Disord. 2017; 32(7):1047–1055. PMID: 28712121.
Article24. Prasad S, Saini J, Yadav R, Pal PK. Motor asymmetry and neuromelanin imaging: concordance in Parkinson's disease. Parkinsonism Relat Disord. 2018; 53:28–32. PMID: 29709506.
Article25. Mendoza-Santiesteban CE, Palma JA, Ortuño-Lizarán I, Cuenca N, Kaufmann H. Pathologic confirmation of retinal ganglion cell loss in multiple system atrophy. Neurology. 2017; 88(23):2233–2235. PMID: 28490649.
Article26. Sari ES, Koc R, Yazici A, Sahin G, Ermis SS. Ganglion cell-inner plexiform layer thickness in patients with Parkinson disease and association with disease severity and duration. J Neuroophthalmol. 2015; 35(2):117–121. PMID: 25485861.
Article27. Ahn J, Lee JY, Kim TW, Yoon EJ, Oh S, Kim YK, et al. Retinal thinning associates with nigral dopaminergic loss in de novo Parkinson disease. Neurology. 2018; 91(11):e1003–12. PMID: 30111550.
Article28. Jiménez B, Ascaso FJ, Cristóbal JA, López del Val J. Development of a prediction formula of Parkinson disease severity by optical coherence tomography. Mov Disord. 2014; 29(1):68–74. PMID: 24458320.
Article29. Satue M, Garcia-Martin E, Fuertes I, Otin S, Alarcia R, Herrero R, et al. Use of Fourier-domain OCT to detect retinal nerve fiber layer degeneration in Parkinson's disease patients. Eye (Lond). 2013; 27(4):507–514. PMID: 23429414.
Article30. Yust-Katz S, Tesler D, Treves TA, Melamed E, Djaldetti R. Handedness as a predictor of side of onset of Parkinson's disease. Parkinsonism Relat Disord. 2008; 14(8):633–635. PMID: 18346926.
Article31. Archibald NK, Clarke MP, Mosimann UP, Burn DJ. Retinal thickness in Parkinson's disease. Parkinsonism Relat Disord. 2011; 17(6):431–436. PMID: 21454118.
Article32. Hajee ME, March WF, Lazzaro DR, Wolintz AH, Shrier EM, Glazman S, et al. Inner retinal layer thinning in Parkinson disease. Arch Ophthalmol. 2009; 127(6):737–741. PMID: 19506190.
Article33. Mailankody P, Battu R, Khanna A, Lenka A, Yadav R, Pal PK. Optical coherence tomography as a tool to evaluate retinal changes in Parkinson's disease. Parkinsonism Relat Disord. 2015; 21(10):1164–1169. PMID: 26297381.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Retinal Thickness and Its Interocular Asymmetry Between Parkinson’s Disease and Drug-Induced Parkinsonism
- Correlation between Interocular Asymmetry of Corneal Hysteresis and Visual Field Defect in Glaucoma
- The Analysis of Peripapillary RNFL, Macula and Macular Ganglion Cell Layer Thickness in Patients with Monocular Amblyopia Using SD-OCT
- Association between Parkinson's Disease and Helicobacter Pylori
- Asymmetry Analysis of the Retinal Nerve Fiber Layer Thickness in Normal Eyes using Optical Coherence Tomography