Ewha Med J.
2021 Jan;44(1):11-18. 10.12771/emj.2021.44.1.11.
New Molecular Targeted Therapy of Metastatic Colorectal Cancer
- Affiliations
-
- 1Division of Hemato-Oncology, Ewha Womans University Mokdong Hospital, Seoul, Korea
- KMID: 2512406
- DOI: http://doi.org/10.12771/emj.2021.44.1.11
Abstract
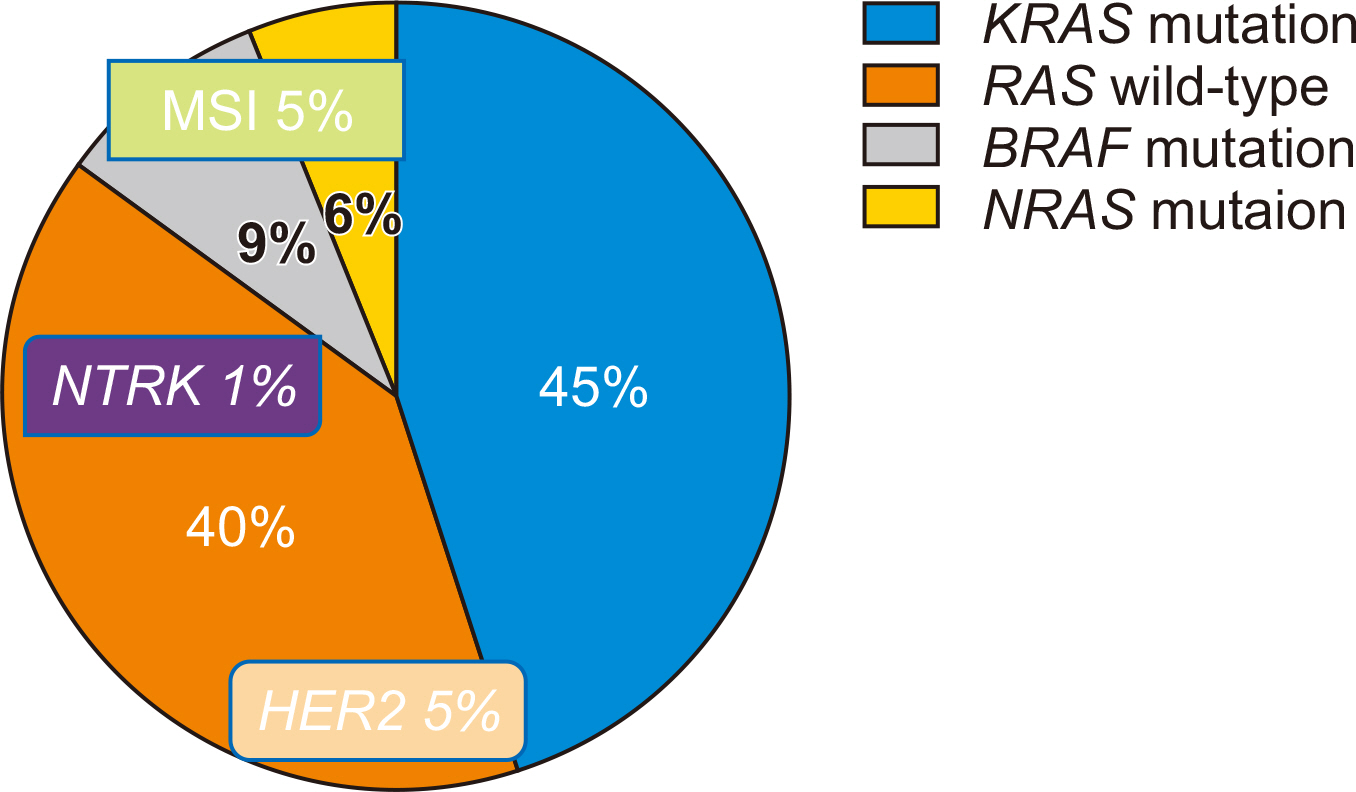
- Over the past decade, substantial advances have been made in the individualization of therapeutic strategies for metastatic colorectal cancer (mCRC). Treatment strategies have been developed and classified according to their molecular and genetic characteristics based on predictive biomarkers such as microsatellite instability, RAS and BRAF mutations, HER2 amplification, or NTRK fusions. As molecular and genetic predictive tests are routinely performed, new challenges for mCRC treatment strategies are allowed. For patients responding to anti-epithelial growth factor receptor treatments, expanded biomarkers panels enable customized treatment to be selected and the optimal treatment can be determined. Patients with mCRC with the BRAFV600E mutation who did not have effective targeted treatments have effective therapeutic options. Attractive but rare targets, such as HER2 amplification and NTRK fusions, could be a breakthrough and the use of immune checkpoint inhibitors in patients with mismatch repair deficiency/microsatellite instability is the striking revolution. In this review, we summarize the current landscape of targeted therapies for mCRC patients, with a focus on new developments for epithelial growth factor receptor blockade and emerging biomarkers.
Keyword
Figure
Reference
-
1. Korea Central Cancer Registry. 2021. Annual report of cancer registry in 2018. Korea Central Cancer Registry;Goyang:2. Lievre A, Bachet JB, Le Corre D, Boige V, Landi B, Emile JF, et al. 2006; KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res. 66:3992–3995. DOI: 10.1158/0008-5472.CAN-06-0191. PMID: 16618717.
Article3. Cercek A, Braghiroli MI, Chou JF, Hechtman JF, Kemeny N, Saltz L, et al. 2017; Clinical features and outcomes of patients with colorectal cancers harboring NRAS mutations. Clin Cancer Res. 23:4753–4760. DOI: 10.1158/1078-0432.CCR-17-0400. PMID: 28446505. PMCID: PMC5788017.
Article4. Pietrantonio F, Vernieri C, Siravegna G, Mennitto A, Berenato R, Perrone F, et al. 2017; Heterogeneity of acquired resistance to anti-EGFR monoclonal antibodies in patients with metastatic colorectal cancer. Clin Cancer Res. 23:2414–2422. DOI: 10.1158/1078-0432.CCR-16-1863. PMID: 27780856.
Article5. Taieb J, Tabernero J, Mini E, Subtil F, Folprecht G, Van Laethem JL, et al. 2014; Oxaliplatin, fluorouracil, and leucovorin with or without cetuximab in patients with resected stage III colon cancer (PETACC-8): an open-label, randomised phase 3 trial. Lancet Oncol. 15:862–873. DOI: 10.1016/S1470-2045(14)70227-X. PMID: 24928083.
Article6. Bridgewater JA, Pugh SA, Maishman T, Eminton Z, Mellor J, Whitehead A, et al. 2020; Systemic chemotherapy with or without cetuximab in patients with resectable colorectal liver metastasis (New EPOC): long-term results of a multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 21:398–411. DOI: 10.1016/S1470-2045(19)30798-3. PMID: 32014119. PMCID: PMC7052737.7. Gholami S, Grothey A. 2020; EGFR antibodies in resectable metastatic colorectal liver metastasis: more harm than benefit? Lancet Oncol. 21:324–326. DOI: 10.1016/S1470-2045(20)30003-6. PMID: 32014120.
Article8. Van Cutsem E, Kohne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, et al. 2009; Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 360:1408–1417. DOI: 10.1056/NEJMoa0805019. PMID: 19339720.
Article9. Tveit KM, Guren T, Glimelius B, Pfeiffer P, Sorbye H, Pyrhonen S, et al. 2012; Phase III trial of cetuximab with continuous or intermittent fluorouracil, leucovorin, and oxaliplatin (Nordic FLOX) versus FLOX alone in first-line treatment of metastatic colorectal cancer: the NORDIC-VII study. J Clin Oncol. 30:1755–1762. DOI: 10.1200/JCO.2011.38.0915. PMID: 22473155.
Article10. Maughan TS, Adams RA, Smith CG, Meade AM, Seymour MT, Wilson RH, et al. 2011; Addition of cetuximab to oxaliplatin-based first-line combination chemotherapy for treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet. 377:2103–2114. DOI: 10.1016/S0140-6736(11)60613-2. PMID: 21641636. PMCID: PMC3159415.11. Qin S, Li J, Wang L, Xu J, Cheng Y, Bai Y, et al. 2018; Efficacy and tolerability of first-line cetuximab plus leucovorin, fluorouracil, and oxaliplatin (FOLFOX-4) versus FOLFOX-4 in patients with RAS wild-type metastatic colorectal cancer: the open-label, randomized, phase III TAILOR trial. J Clin Oncol. 36:3031–3039. DOI: 10.1200/JCO.2018.78.3183. PMID: 30199311. PMCID: PMC6324088.
Article12. Venook AP. 2017; Right-sided vs left-sided colorectal cancer. Clin Adv Hematol Oncol. 15:22–24. PMID: 28212365.13. Arnold D, Lueza B, Douillard JY, Peeters M, Lenz HJ, Venook A, et al. 2017; Prognostic and predictive value of primary tumour side in patients with RAS wild-type metastatic colorectal cancer treated with chemotherapy and EGFR directed antibodies in six randomized trials. Ann Oncol. 28:1713–1729. DOI: 10.1093/annonc/mdx175. PMID: 28407110. PMCID: PMC6246616.
Article14. Yin J, Cohen R, Jin Z, Liu H, Pederson L, Adams R, et al. 2020; Prognostic and predictive impact of primary tumor sidedness in first-line trials for advanced colorectal cancer: an analysis of 7,828 patients in the ARCAD database. J Clin Oncol. 38(4 suppl):188. DOI: 10.1200/JCO.2020.38.4_suppl.188.
Article15. Venderbosch S, Nagtegaal ID, Maughan TS, Smith CG, Cheadle JP, Fisher D, et al. 2014; Mismatch repair status and BRAF mutation status in metastatic colorectal cancer patients: a pooled analysis of the CAIRO, CAIRO2, COIN, and FOCUS studies. Clin Cancer Res. 20:5322–5330. DOI: 10.1158/1078-0432.CCR-14-0332. PMID: 25139339. PMCID: PMC4201568.
Article16. Yaeger R, Kotani D, Mondaca S, Parikh AR, Bando H, Van Seventer EE, et al. 2019; Response to anti-EGFR therapy in patients with BRAF non-V600-mutant metastatic colorectal cancer. Clin Cancer Res. 25:7089–7097. DOI: 10.1158/1078-0432.CCR-19-2004. PMID: 31515458. PMCID: PMC6891165.
Article17. Tran B, Kopetz S, Tie J, Gibbs P, Jiang ZQ, Lieu CH, et al. 2011; Impact of BRAF mutation and microsatellite instability on the pattern of metastatic spread and prognosis in metastatic colorectal cancer. Cancer. 117:4623–4632. DOI: 10.1002/cncr.26086. PMID: 21456008. PMCID: PMC4257471.
Article18. Loupakis F, Cremolini C, Antoniotti C, Lonardi S, Ronzoni M, Zaniboni A, et al. 2015; FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as initial treatment for metastatic colorectal cancer (TRIBE study): updated survival results and final molecular subgroups analyses. J Clin Oncol. 33(15 suppl):3510. DOI: 10.1200/jco.2015.33.15_suppl.3510.
Article19. Cremolini C, Antoniotti C, Lonardi S, Rossini D, Morano F, Cordio S, et al. 2019; Updated results of TRIBE2, a phase III, randomized strategy study by GONO in the 1st- and 2nd-line treatment of unresectable mCRC. Ann Oncol. 30:iv154. DOI: 10.1093/annonc/mdz183.005.
Article20. Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, et al. 2004; Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 350:2335–2342. DOI: 10.1056/NEJMoa032691. PMID: 15175435.
Article21. Price TJ, Hardingham JE, Lee CK, Weickhardt A, Townsend AR, Wrin JW, et al. 2011; Impact of KRAS and BRAF gene mutation status on outcomes from the phase III AGITG MAX trial of capecitabine alone or in combination with bevacizumab and mitomycin in advanced colorectal cancer. J Clin Oncol. 29:2675–2682. DOI: 10.1200/JCO.2010.34.5520. PMID: 21646616.
Article22. Yoshino T, Portnoy DC, Obermannova R, Bodoky G, Prausova J, Garcia-Carbonero R, et al. 2019; Biomarker analysis beyond angiogenesis: RAS/RAF mutation status, tumour sidedness, and second-line ramucirumab efficacy in patients with metastatic colorectal carcinoma from RAISE-a global phase III study. Ann Oncol. 30:124–131. DOI: 10.1093/annonc/mdy461. PMID: 30339194. PMCID: PMC6336001.
Article23. Wirapati P, Pomella V, Vandenbosch B, Kerr P, Maiello E, Grahame MJ, et al. 2017; Velour trial biomarkers update: impact of RAS, BRAF, and sidedness on aflibercept activity. Ann Oncol. 28:iii151–iii152. DOI: 10.1093/annonc/mdx302.004.
Article24. Gelsomino F, Casadei-Gardini A, Rossini D, Boccaccino A, Masi G, Cremolini C, et al. 2020; The role of anti-angiogenics in pre-treated metastatic BRAF-mutant colorectal cancer: a pooled analysis. Cancers (Basel). 12:1022. DOI: 10.3390/cancers12041022. PMID: 32326305. PMCID: PMC7226019.25. Pietrantonio F, Petrelli F, Coinu A, Di Bartolomeo M, Borgonovo K, Maggi C, et al. 2015; Predictive role of BRAF mutations in patients with advanced colorectal cancer receiving cetuximab and panitumumab: a meta-analysis. Eur J Cancer. 51:587–594. DOI: 10.1016/j.ejca.2015.01.054. PMID: 25673558.
Article26. Rowland A, Dias MM, Wiese MD, Kichenadasse G, McKinnon RA, Karapetis CS, et al. 2015; Meta-analysis of BRAF mutation as a predictive biomarker of benefit from anti-EGFR monoclonal antibody therapy for RAS wild-type metastatic colorectal cancer. Br J Cancer. 112:1888–1894. DOI: 10.1038/bjc.2015.173. PMID: 25989278. PMCID: PMC4580381.27. Stintzing S, Miller-Phillips L, Modest DP, Fischer von Weikersthal L, Decker T, Kiani A, et al. 2017; Impact of BRAF and RAS mutations on first-line efficacy of FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab: analysis of the FIRE-3 (AIO KRK-0306) study. Eur J Cancer. 79:50–60. DOI: 10.1016/j.ejca.2017.03.023. PMID: 28463756.
Article28. Modest DP, Martens UM, Riera-Knorrenschild J, Greeve J, Florschutz A, Wessendorf S, et al. 2019; FOLFOXIRI plus panitumumab as first-line treatment of RAS wild-type metastatic colorectal cancer: the randomized, open-label, phase II VOLFI Study (AIO KRK0109). J Clin Oncol. 37:3401–3411. DOI: 10.1200/JCO.19.01340. PMID: 31609637.29. Corcoran RB, Ebi H, Turke AB, Coffee EM, Nishino M, Cogdill AP, et al. 2012; EGFR-mediated re-activation of MAPK signaling contributes to insensitivity of BRAF mutant colorectal cancers to RAF inhibition with vemurafenib. Cancer Discov. 2:227–235. DOI: 10.1158/2159-8290.CD-11-0341. PMID: 22448344. PMCID: PMC3308191.30. Hong DS, Morris VK, El Osta B, Sorokin AV, Janku F, Fu S, et al. 2016; Phase IB study of vemurafenib in combination with irinotecan and cetuximab in patients with metastatic colorectal cancer with BRAFV600E mutation. Cancer Discov. 6:1352–1365. DOI: 10.1158/2159-8290.CD-16-0050. PMID: 27729313. PMCID: PMC5562357.31. Kopetz S, Grothey A, Yaeger R, Van Cutsem E, Desai J, Yoshino T, et al. 2019; Encorafenib, binimetinib, and cetuximab in BRAF V600E-mutated colorectal cancer. N Engl J Med. 381:1632–1643. DOI: 10.1056/NEJMoa1908075. PMID: 31566309.32. Kopetz S, Grothey A, Van Cutsem E, Yaeger R, Wasan HS, Yoshino T, et al. 2020; Encorafenib plus cetuximab with or without binimetinib for BRAF V600E-mutant metastatic colorectal cancer: quality-of-life results from a randomized, three-arm, phase III study versus the choice of either irinotecan or FOLFIRI plus cetuximab (BEACON CRC). J Clin Oncol. 38(15 suppl):4039. DOI: 10.1200/JCO.2020.38.15_suppl.4039.33. Nagasaka M, Li Y, Sukari A, Ou SI, Al-Hallak MN, Azmi AS. 2020; KRAS G12C Game of Thrones, which direct KRAS inhibitor will claim the iron throne? Cancer Treat Rev. 84:101974. DOI: 10.1016/j.ctrv.2020.101974. PMID: 32014824. PMCID: PMC7041424.
Article34. Fakih M, Desai J, Kuboki Y, Strickler JH, Price TJ, Durm GA, et al. 2020; CodeBreak 100: activity of AMG 510, a novel small molecule inhibitor of KRASG12C, in patients with advanced colorectal cancer. J Clin Oncol. 38(15 suppl):4018.35. Colle R, Cohen R, Cochereau D, Duval A, Lascols O, Lopez-Trabada D, et al. 2017; Immunotherapy and patients treated for cancer with microsatellite instability. Bull Cancer. 104:42–51. DOI: 10.1016/j.bulcan.2016.11.006. PMID: 27979364.
Article36. Innocenti F, Ou FS, Qu X, Zemla TJ, Niedzwiecki D, Tam R, et al. 2019; Mutational analysis of patients with colorectal cancer in CALGB/SWOG 80405 identifies new roles of microsatellite instability and tumor mutational burden for patient outcome. J Clin Oncol. 37:1217–1227. DOI: 10.1200/JCO.18.01798. PMID: 30865548. PMCID: PMC6506418.
Article37. Marisa L, Svrcek M, Collura A, Becht E, Cervera P, Wanherdrick K, et al. 2018; The balance between cytotoxic T-cell lymphocytes and immune checkpoint expression in the prognosis of colon tumors. J Natl Cancer Inst. 110:66–77. DOI: 10.1093/jnci/djx136. PMID: 28922790.
Article38. Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, et al. 2017; Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 357:409–413. DOI: 10.1126/science.aan6733. PMID: 28596308. PMCID: PMC5576142.39. Lenz HJ, Van Cutsem E, Limon ML, Wong KY, Hendlisz A, Aglietta M, et al. 2018; Durable clinical benefit with nivolumab (NIVO) plus low-dose ipilimumab (IPI) as first-line therapy in microsatellite instability-high/mismatch repair deficient (MSI-H/dMMR) metastatic colorectal cancer (mCRC). Ann Oncol. 29:viii714. DOI: 10.1093/annonc/mdy424.019.
Article40. Andre T, Shiu KK, Kim TW, Jensen BV, Jensen LH, Punt CJ, et al. 2020; Pembrolizumab versus chemotherapy for microsatellite instability-high/mismatch repair deficient metastatic colorectal cancer: the phase 3 KEYNOTE-177 Study. J Clin Oncol. 38(18 suppl):LBA4. DOI: 10.1200/JCO.2020.38.18_suppl.LBA4.
Article41. Chalabi M, Fanchi LF, Dijkstra KK, Aalbers AG, Sikorska K, et al. , Van den Berg JG. 2020; Neoadjuvant immunotherapy leads to pathological responses in MMR-proficient and MMR-deficient early-stage colon cancers. Nat Med. 26:566–576. DOI: 10.1038/s41591-020-0805-8. PMID: 32251400.
Article42. Jonker DJ, O'Callaghan CJ, Karapetis CS, Zalcberg JR, Tu D, Au HJ, et al. 2007; Cetuximab for the treatment of colorectal cancer. N Engl J Med. 357:2040–2048. DOI: 10.1056/NEJMoa071834. PMID: 18003960.
Article43. Shin DS, Zaretsky JM, Escuin-Ordinas H, Garcia-Diaz A, Hu-Lieskovan S, Kalbasi A, et al. 2017; Primary resistance to PD-1 blockade mediated by JAK1/2 mutations. Cancer Discov. 7:188–201. DOI: 10.1158/2159-8290.CD-16-1223. PMID: 27903500. PMCID: PMC5296316.44. Loupakis F, Depetris I, Biason P, Intini R, Prete AA, Leone F, et al. 2020; Prediction of benefit from checkpoint inhibitors in mismatch repair deficient metastatic colorectal cancer: role of tumor infiltrating lymphocytes. Oncologist. 25:481–487. DOI: 10.1634/theoncologist.2019-0611. PMID: 31967692. PMCID: PMC7288636.
Article45. Ross JS, Fakih M, Ali SM, Elvin JA, Schrock AB, Suh J, et al. 2018; Targeting HER2 in colorectal cancer: the landscape of amplification and short variant mutations in ERBB2 and ERBB3. Cancer. 124:1358–1373. DOI: 10.1002/cncr.31125. PMID: 29338072. PMCID: PMC5900732.46. Sartore-Bianchi A, Trusolino L, Martino C, Bencardino K, Lonardi S, Bergamo F, et al. 2016; Dual-targeted therapy with trastuzumab and lapatinib in treatment-refractory, KRAS codon 12/13 wild-type, HER2-positive metastatic colorectal cancer (HERACLES): a proof-of-concept, multicentre, open-label, phase 2 trial. Lancet Oncol. 17:738–746. DOI: 10.1016/S1470-2045(16)00150-9. PMID: 27108243.47. Valtorta E, Martino C, Sartore-Bianchi A, Penaullt-Llorca F, Viale G, Risio M, et al. 2015; Assessment of a HER2 scoring system for colorectal cancer: results from a validation study. Mod Pathol. 28:1481–1491. DOI: 10.1038/modpathol.2015.98. PMID: 26449765.
Article48. Meric-Bernstam F, Hurwitz H, Raghav KPS, McWilliams RR, Fakih M, VanderWalde A, et al. 2019; Pertuzumab plus trastuzumab for HER2-amplified metastatic colorectal cancer (MyPathway): an updated report from a multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol. 20:518–530. DOI: 10.1016/S1470-2045(18)30904-5. PMID: 30857956. PMCID: PMC6781620.
Article49. Strickler JH, Zemla T, Ou FS, Cercek A, Wu C, Sanchez FA, et al. 2019; Trastuzumab and tucatinib for the treatment of HER2 amplified metastatic colorectal cancer (mCRC): initial results from the MOUNTAINEER trial. Ann Oncol. 30:v200. DOI: 10.1093/annonc/mdz246.005.
Article50. Drilon A, Laetsch TW, Kummar S, DuBois SG, Lassen UN, Demetri GD, et al. 2018; Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children. N Engl J Med. 378:731–739. DOI: 10.1056/NEJMoa1714448. PMID: 29466156. PMCID: PMC5857389.51. Drilon A, Siena S, Ou SI, Patel M, Ahn MJ, Lee J, et al. 2017; Safety and antitumor activity of the multitargeted Pan-TRK, ROS1, and ALK inhibitor entrectinib: combined results from two phase I trials (ALKA-372-001 and STARTRK-1). Cancer Discov. 7:400–409. DOI: 10.1158/2159-8290.CD-16-1237. PMID: 28183697. PMCID: PMC5380583.52. Yoshino T, Pentheroudakis G, Mishima S, Overman MJ, Yeh KH, Baba E, et al. 2020; JSCO-ESMO-ASCO-JSMO-TOS: international expert consensus recommendations for tumour-agnostic treatments in patients with solid tumours with microsatellite instability or NTRK fusions. Ann Oncol. 31:861–872. DOI: 10.1016/j.annonc.2020.03.299. PMID: 32272210.53. Pietrantonio F, Di Nicolantonio F, Schrock AB, Lee J, Tejpar S, Sartore-Bianchi A, et al. 2017; ALK, ROS1, and NTRK rearrangements in metastatic colorectal cancer. J Natl Cancer Inst. 109:djx0f9. DOI: 10.1093/jnci/djx089. PMID: 29370427.54. Cocco E, Benhamida J, Middha S, Zehir A, Mullaney K, Shia J, et al. 2019; Colorectal carcinomas containing hypermethylated MLH1 promoter and wild-type BRAF/KRAS are enriched for targetable kinase fusions. Cancer Res. 79:1047–1053. DOI: 10.1158/0008-5472.CAN-18-3126. PMID: 30643016. PMCID: PMC6420871.
Article55. Cohen R, Pudlarz T, Delattre JF, Colle R, Andre T. 2020; Molecular targets for the treatment of metastatic colorectal cancer. Cancers (Basel). 12:2350. DOI: 10.3390/cancers12092350. PMID: 32825275. PMCID: PMC7563268.
Article