Anesth Pain Med.
2021 Jan;16(1):81-95. 10.17085/apm.20078.
Prolotherapy for the patients with chronic musculoskeletal pain: systematic review and meta-analysis
- Affiliations
-
- 1Department of Anesthesiology and Pain Medicine, Sanggye Paik Hospital, Inje University College of Medicine, Seoul, Korea
- KMID: 2512286
- DOI: http://doi.org/10.17085/apm.20078
Abstract
- Background
Prolotherapy, which stimulates the healing of loosened ligaments and tendons, is a cost-effective and safe treatment modality for chronic musculoskeletal pain. Its benefits may be affected by injection protocols, comparative regimens, and evaluation scales. The aim of this study was to determine the effectiveness of dextrose prolotherapy as a long-term treatment for chronic musculoskeletal pain.
Methods
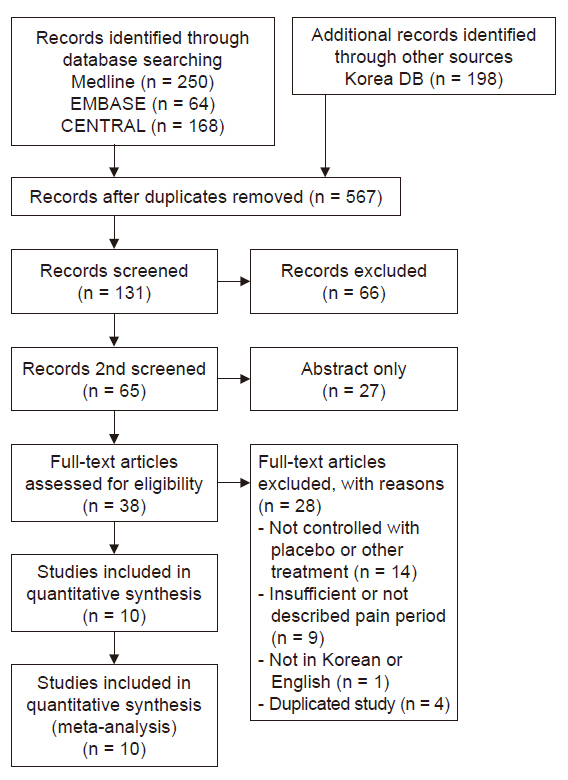
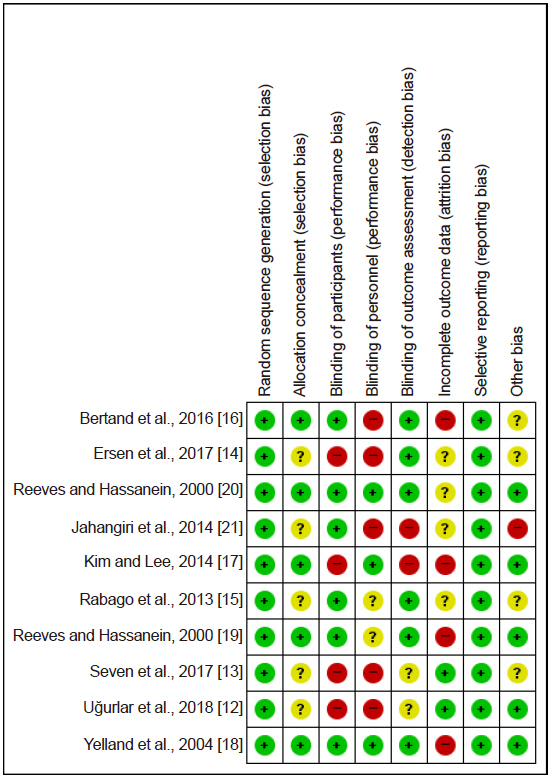
Medline, Embase, Cochrane Central, KoreaMed, and KMbase databases were searched for studies published up to March 2019. We included randomized controlled trials which compared the effect of dextrose prolotherapy with that of other therapies such as exercise, saline, platelet-rich plasma, and steroid injection. The primary outcome was pain score change during daily life.
Results
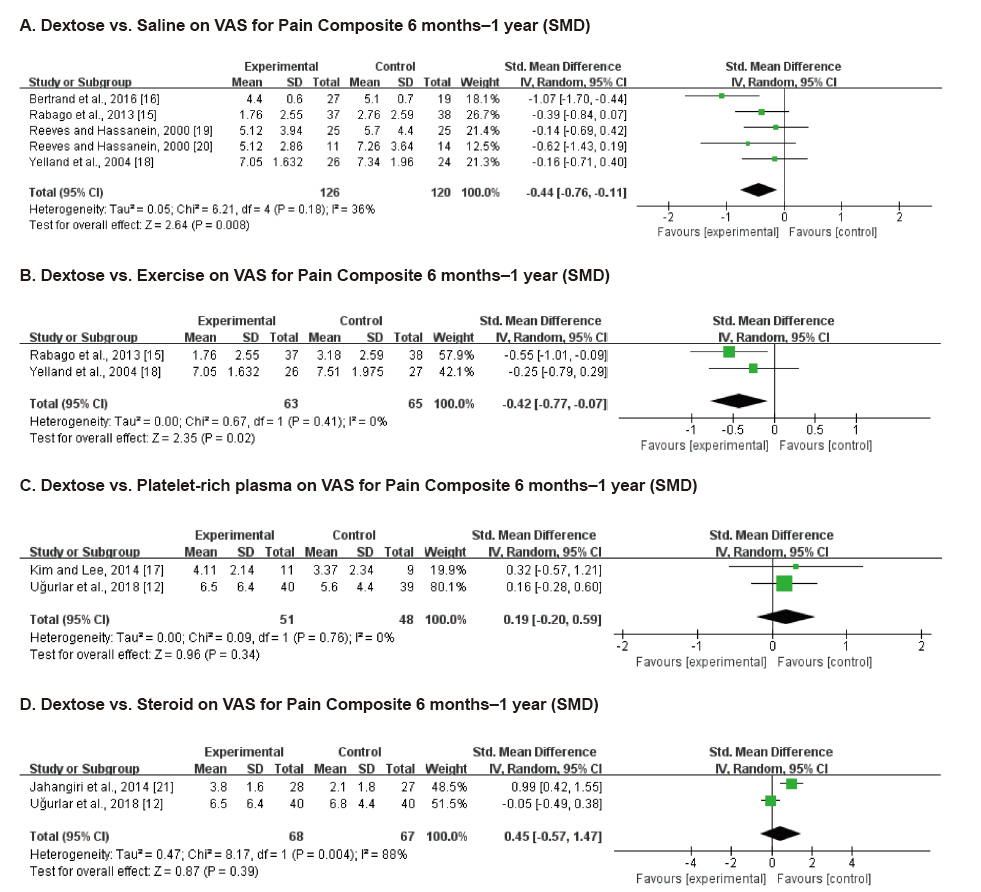
Ten studies involving 750 participants were included in the final analysis. Pain scores from 6 months to 1 year after dextrose prolotherapy were significantly reduced compared to saline injection (standardized mean difference [SMD] –0.44; 95% confidence interval [CI] –0.76 to –0.11, P = 0.008) and exercise (SMD –0.42; 95% CI –0.77 to –0.07, P = 0.02). Prolotherapy yielded results similar to platelet-rich plasma or steroid injection, that it showed no significant difference in pain score.
Conclusions
Dextrose prolotherapy is more effective in the treatment of chronic pain compared to saline injection or exercise. Its effect was comparable to that of platelet-rich plasma or steroid injection. Adequately powered, homogeneous, and longer-term trials are needed to better elucidate the efficacy of prolotherapy.
Figure
Reference
-
1. Institute of Medicine of the National Academies. Relieving pain in America: a blueprint for transforming prevention, care, education, and research. Washington, D.C.: National Academies Press;2011. p. 382.2. Hung CY, Hsiao MY, Chang KV, Han DS, Wang TG. Comparative effectiveness of dextrose prolotherapy versus control injections and exercise in the management of osteoarthritis pain: a systematic review and meta-analysis. J Pain Res. 2016; 9:847–57.3. Ayhan E, Kesmezacar H, Akgun I. Intraarticular injections (corticosteroid, hyaluronic acid, platelet rich plasma) for the knee osteoarthritis. World J Orthop. 2014; 5:351–61.4. Bellamy N, Campbell J, Robinson V, Gee T, Bourne R, Wells G. Intraarticular corticosteroid for treatment of osteoarthritis of the knee. Cochrane Database Syst Rev. 2006; (2):CD005328.5. Aufiero D, Vincent H, Sampson S, Bodor M. Regenerative injection treatment in the spine: review and case series with platelet rich plasma. J Stem Cells Res Rev Rep. 2015; 2:1019.6. Linetsky FS, Manchikanti L. Regenerative injection therapy for axial pain. Tech Reg Anesth Pain Manag. 2005; 9:40–9.7. Adams E. Bibliography: prolotherapy for musculoskeletal pain. Boston: Veterans;2008.8. Goswami A. Prolotherapy. J Pain Palliat Care Pharmacother. 2012; 26:376–8.9. Nair LS. Prolotherapy for tissue repair. Transl Res. 2011; 158:129–31.10. Rabago D, Best TM, Beamsley M, Patterson J. A systematic review of prolotherapy for chronic musculoskeletal pain. Clin J Sport Med. 2005; 15:376–80.11. ; Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011; 343:d5928.12. Uğurlar M, Sönmez MM, Uğurlar ÖY, Adıyeke L, Yıldırım H, Eren OT. Effectiveness of four different treatment modalities in the treatment of chronic plantar fasciitis during a 36-month follow-up period: a randomized controlled trial. J Foot Ankle Surg. 2018; 57:913–8.13. Seven MM, Ersen O, Akpancar S, Ozkan H, Turkkan S, Yıldız Y, et al. Effectiveness of prolotherapy in the treatment of chronic rotator cuff lesions. Orthop Traumatol Surg Res. 2017; 103:427–33.14. Ersen Ö, Koca K, Akpancar S, Seven MM, Akyıldız F, Yıldız Y, et al. A randomized-controlled trial of prolotherapy injections in the treatment of plantar fasciitis. Turk J Phys Med Rehabil. 2017; 64:59–65.15. Rabago D, Patterson JJ, Mundt M, Kijowski R, Grettie J, Segal NA, et al. Dextrose prolotherapy for knee osteoarthritis: a randomized controlled trial. Ann Fam Med. 2013; 11:229–37.16. Bertrand H, Reeves KD, Bennett CJ, Bicknell S, Cheng AL. Dextrose prolotherapy versus control injections in painful rotator cuff tendinopathy. Arch Phys Med Rehabil. 2016; 97:17–25.17. Kim E, Lee JH. Autologous platelet-rich plasma versus dextrose prolotherapy for the treatment of chronic recalcitrant plantar fasciitis. PM R. 2014; 6:152–8.18. Yelland MJ, Glasziou PP, Bogduk N, Schluter PJ, McKernon M. Prolotherapy injections, saline injections, and exercises for chronic low-back pain: a randomized trial. Spine (Phila Pa 1976). 2004; 29:9–16.19. Reeves KD, Hassanein K. Randomized, prospective, placebo-controlled double-blind study of dextrose prolotherapy for osteoarthritic thumb and finger (DIP, PIP, and trapeziometacarpal) joints: evidence of clinical efficacy. J Altern Complement Med. 2000; 6:311–20.20. Reeves KD, Hassanein K. Randomized prospective double-blind placebo-controlled study of dextrose prolotherapy for knee osteoarthritis with or without ACL laxity. Altern Ther Health Med. 2000; 6:68–74. , 77-80.21. Jahangiri A, Moghaddam FR, Najafi S. Hypertonic dextrose versus corticosteroid local injection for the treatment of osteoarthritis in the first carpometacarpal joint: a double-blind randomized clinical trial. J Orthop Sci. 2014; 19:737–43.22. Sit RW, Chung VC, Reeves KD, Rabago D, Chan KK, Chan DC, et al. Hypertonic dextrose injections (prolotherapy) in the treatment of symptomatic knee osteoarthritis: a systematic review and meta-analysis. Sci Rep. 2016; 6:25247.23. Paavola M, Kannus P, Järvinen TA, Järvinen TL, Józsa L, Järvinen M. Treatment of tendon disorders. Is there a role for corticosteroid injection? Foot Ankle Clin. 2002; 7:501–13.24. Dean BJ, Lostis E, Oakley T, Rombach I, Morrey ME, Carr AJ. The risks and benefits of glucocorticoid treatment for tendinopathy: a systematic review of the effects of local glucocorticoid on tendon. Semin Arthritis Rheum. 2014; 43:570–6.25. Tempfer H, Gehwolf R, Lehner C, Wagner A, Mtsariashvili M, Bauer HC, et al. Effects of crystalline glucocorticoid triamcinolone acetonide on cultered human supraspinatus tendon cells. Acta Orthop. 2009; 80:357–62.26. Stannard JP, Bucknell AL. Rupture of the triceps tendon associated with steroid injections. Am J Sports Med. 1993; 21:482–5.27. Scarpone M, Rabago DP, Zgierska A, Arbogast G, Snell E. The efficacy of prolotherapy for lateral epicondylosis: a pilot study. Clin J Sport Med. 2008; 18:248–54.28. Okuda Y, Adrogue HJ, Nakajima T, Mizutani M, Asano M, Tachi Y, et al. Increased production of PDGF by angiotensin and high glucose in human vascular endothelium. Life Sci. 1996; 59:1455–61.29. Oh JH, Ha H, Yu MR, Lee HB. Sequential effects of high glucose on mesangial cell transforming growth factor-beta 1 and fibronectin synthesis. Kidney Int. 1998; 54:1872–8.30. Di Paolo S, Gesualdo L, Ranieri E, Grandaliano G, Schena FP. High glucose concentration induces the overexpression of transforming growth factor-beta through the activation of a platelet-derived growth factor loop in human mesangial cells. Am J Pathol. 1996; 149:2095–106.31. Kampa RJ, Connell DA. Treatment of tendinopathy: is there a role for autologous whole blood and platelet rich plasma injection? Int J Clin Pract. 2010; 64:1813–23.32. Sampson S, Gerhardt M, Mandelbaum B. Platelet rich plasma injection grafts for musculoskeletal injuries: a review. Curr Rev Musculoskelet Med. 2008; 1:165–74.33. Foster TE, Puskas BL, Mandelbaum BR, Gerhardt MB, Rodeo SA. Platelet-rich plasma: from basic science to clinical applications. Am J Sports Med. 2009; 37:2259–72.34. de Mos M, van der Windt AE, Jahr H, van Schie HT, Weinans H, Verhaar JA, et al. Can platelet-rich plasma enhance tendon repair? A cell culture study. Am J Sports Med. 2008; 36:1171–8.35. Jensen KT, Rabago DP, Best TM, Patterson JJ, Vanderby R Jr. Early inflammatory response of knee ligaments to prolotherapy in a rat model. J Orthop Res. 2008; 26:816–23.36. Jensen KT, Rabago DP, Best TM, Patterson JJ, Vanderby R Jr. Response of knee ligaments to prolotherapy in a rat injury model. Am J Sports Med. 2008; 36:1347–57.37. Mei-Dan O, Mann G, Maffulli N. Platelet-rich plasma: any substance into it? Br J Sports Med. 2010; 44:618–9.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Prolotherapy on Chronic Musculoskeletal Disease
- The Effect of Prolotherapy for the Chronic Pain of Musculoskeletal System
- Clinical Experience of Prolotherapy for Chronic Musculoskeletal Disease: A report of 5 cases
- Effect of Auriculotherapy on Musculoskeletal Pain: A Systematic Review and Meta-Analysis
- Prolotherapy