Intest Res.
2021 Jan;19(1):33-44. 10.5217/ir.2019.09175.
Endoscopic molecular imaging in inflammatory bowel disease
- Affiliations
-
- 1Department of Gastroenterology, Veterans Health Service Medical Center, Seoul, Korea
- 2Department of Gastroenterology, Digestive Diseases Research Center, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- KMID: 2512099
- DOI: http://doi.org/10.5217/ir.2019.09175
Abstract
- Molecular imaging is a technique for imaging the processes occurring in a living body at a molecular level in real-time, combining molecular cell biology with advanced imaging technologies using molecular probes and fluorescence. Gastrointestinal endoscopic molecular imaging shows great promise for improving the identification of neoplasms, providing characterization for patient stratification and assessing the response to molecular targeted therapy. In inflammatory bowel disease, endoscopic molecular imaging can be used to assess disease severity and predict therapeutic response and prognosis. Endoscopic molecular imaging is also able to visualize dysplasia in the presence of background inflammation. Several preclinical and clinical trials have evaluated endoscopic molecular imaging; however, this area is just beginning to evolve, and many issues have not been solved yet. In the future, it is expected that endoscopic molecular imaging will be of increasing interest among clinicians as a new technology for the identification and evaluation of colorectal neoplasm and colitis-associated cancer.
Figure
Cited by 2 articles
-
Differentiating between Intestinal Tuberculosis and Crohn’s Disease May Be Complicated by Multidrug-resistant
Mycobacterium tuberculosis
Seung Wook Hong, Sang Hyoung Park, Byong Duk Ye, Suk-Kyun Yang
Ewha Med J. 2021;44(3):93-96. doi: 10.12771/emj.2021.44.3.93.Is Multidrug-resistant Extrapulmonary Tuberculosis Important? If So, What Is Our Strategy?
Seong-Eun Kim
Ewha Med J. 2021;44(4):148-149. doi: 10.12771/emj.2021.44.4.148.
Reference
-
1. Drossman DA. Challenges in the physician-patient relationship: feeling “drained”. Gastroenterology. 2001; 121:1037–1038.
Article2. Matloff JL, Abidi W, Richards-Kortum R, Sauk J, Anandasabapathy S. High-resolution and optical molecular imaging for the early detection of colonic neoplasia. Gastrointest Endosc. 2011; 73:1263–1273.
Article3. van Rijn JC, Reitsma JB, Stoker J, Bossuyt PM, van Deventer SJ, Dekker E. Polyp miss rate determined by tandem colonoscopy: a systematic review. Am J Gastroenterol. 2006; 101:343–350.
Article4. Yalamarthi S, Witherspoon P, McCole D, Auld CD. Missed diagnoses in patients with upper gastrointestinal cancers. Endoscopy. 2004; 36:874–879.
Article5. Dacosta RS, Wilson BC, Marcon NE. New optical technologies for earlier endoscopic diagnosis of premalignant gastrointestinal lesions. J Gastroenterol Hepatol. 2002; 17 Suppl:S85S104.
Article6. Goetz M, Wang TD. Molecular imaging in gastrointestinal endoscopy. Gastroenterology. 2010; 138:828–833.
Article7. Kim JW, Yoon SM, Myung SJ. Molecular imaging of gastrointestinal malignancies. Intest Res. 2011; 9:90–96.
Article8. Kim SY, Myung SJ. Optical molecular imaging for diagnosing intestinal diseases. Clin Endosc. 2013; 46:620–626.
Article9. Shahid MW, Buchner AM, Coron E, et al. Diagnostic accuracy of probe-based confocal laser endomicroscopy in detecting residual colorectal neoplasia after EMR: a prospective study. Gastrointest Endosc. 2012; 75:525–533.
Article10. Shahid MW, Buchner AM, Heckman MG, et al. Diagnostic accuracy of probe-based confocal laser endomicroscopy and narrow band imaging for small colorectal polyps: a feasibility study. Am J Gastroenterol. 2012; 107:231–239.
Article11. Shahid MW, Buchner AM, Raimondo M, Woodward TA, Krishna M, Wallace MB. Accuracy of real-time vs. blinded offline diagnosis of neoplastic colorectal polyps using probebased confocal laser endomicroscopy: a pilot study. Endoscopy. 2012; 44:343–348.
Article12. Wallace MB, Meining A, Canto MI, et al. The safety of intravenous fluorescein for confocal laser endomicroscopy in the gastrointestinal tract. Aliment Pharmacol Ther. 2010; 31:548552.
Article13. Yao J, Wang LV. Recent progress in photoacoustic molecular imaging. Curr Opin Chem Biol. 2018; 45:104–112.
Article14. Yang JM, Favazza C, Chen R, et al. Simultaneous functional photoacoustic and ultrasonic endoscopy of internal organs in vivo. Nat Med. 2012; 18:1297–1302.
Article15. Marten K, Bremer C, Khazaie K, et al. Detection of dysplastic intestinal adenomas using enzyme-sensing molecular beacons in mice. Gastroenterology. 2002; 122:406–414.
Article16. Miller SJ, Joshi BP, Feng Y, Gaustad A, Fearon ER, Wang TD. In vivo fluorescence-based endoscopic detection of colon dysplasia in the mouse using a novel peptide probe. PLoS One. 2011; 6:e17384.
Article17. Joshi BP, Liu Z, Elahi SF, Appelman HD, Wang TD. Near-infrared-labeled peptide multimer functions as phage mimic for high affinity, specific targeting of colonic adenomas in vivo (with videos). Gastrointest Endosc. 2012; 76:1197–1206.
Article18. Zhou L, El-Deiry WS. Multispectral fluorescence imaging. J Nucl Med. 2009; 50:1563–1566.
Article19. Miller SJ, Lee CM, Joshi BP, Gaustad A, Seibel EJ, Wang TD. Targeted detection of murine colonic dysplasia in vivo with flexible multispectral scanning fiber endoscopy. J Biomed Opt. 2012; 17:021103.
Article20. Bae SM, Bae DJ, Do EJ, et al. Multi-spectral fluorescence imaging of colon dysplasia invivo using a multi-spectral endoscopy system. Transl Oncol. 2019; 12:226–235.
Article21. Yoon SM, Myung SJ, Kim IW, et al. Application of near-infrared fluorescence imaging using a polymeric nanoparticlebased probe for the diagnosis and therapeutic monitoring of colon cancer. Dig Dis Sci. 2011; 56:3005–3013.
Article22. Yoon SM, Myung SJ, Ye BD, et al. Near-infrared fluorescence imaging using a protease-specific probe for the detection of colon tumors. Gut Liver. 2010; 4:488–497.
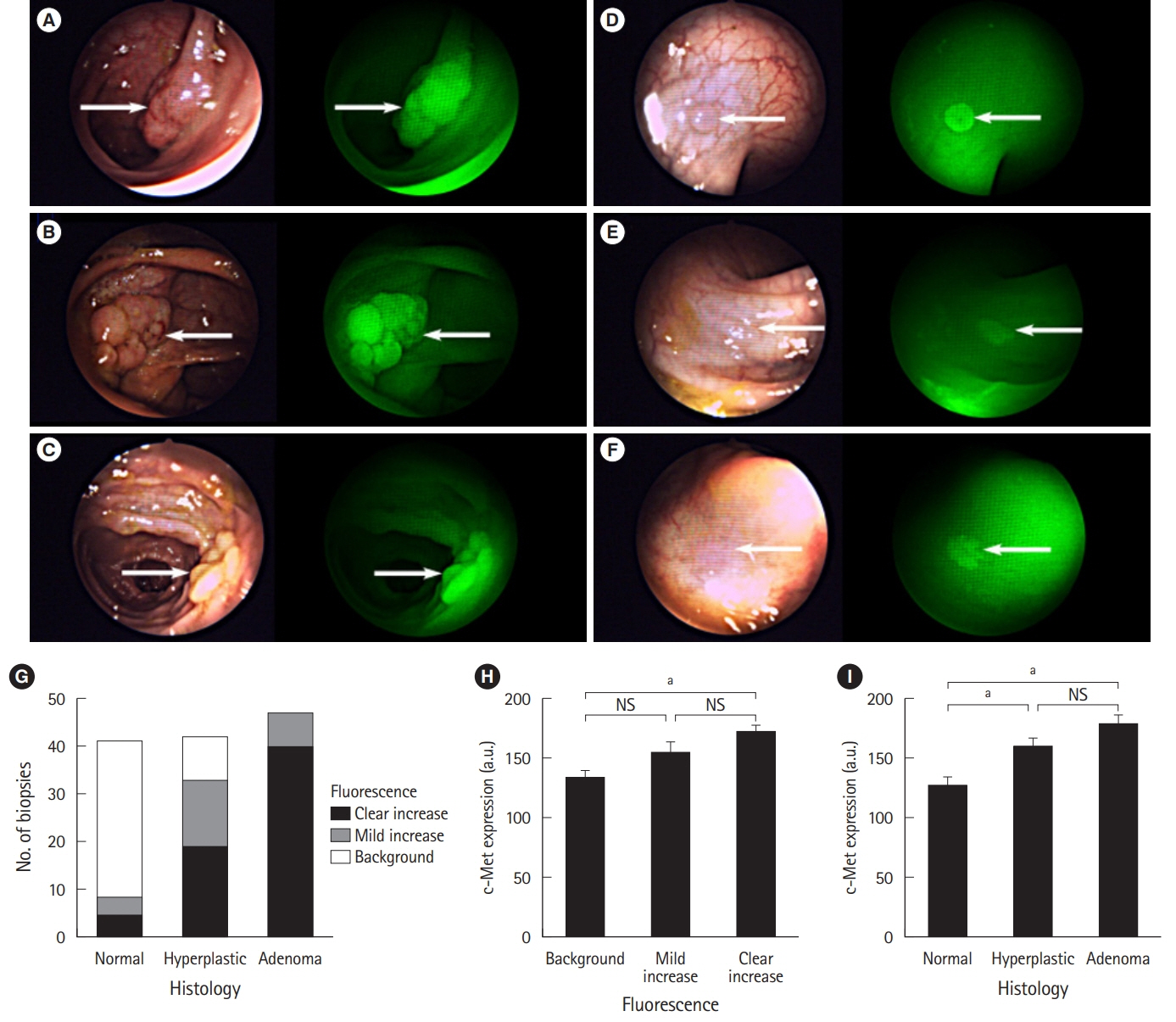
Article23. Burggraaf J, Kamerling IM, Gordon PB, et al. Detection of colorectal polyps in humans using an intravenously administered fluorescent peptide targeted against c-Met. Nat Med. 2015; 21:955–961.
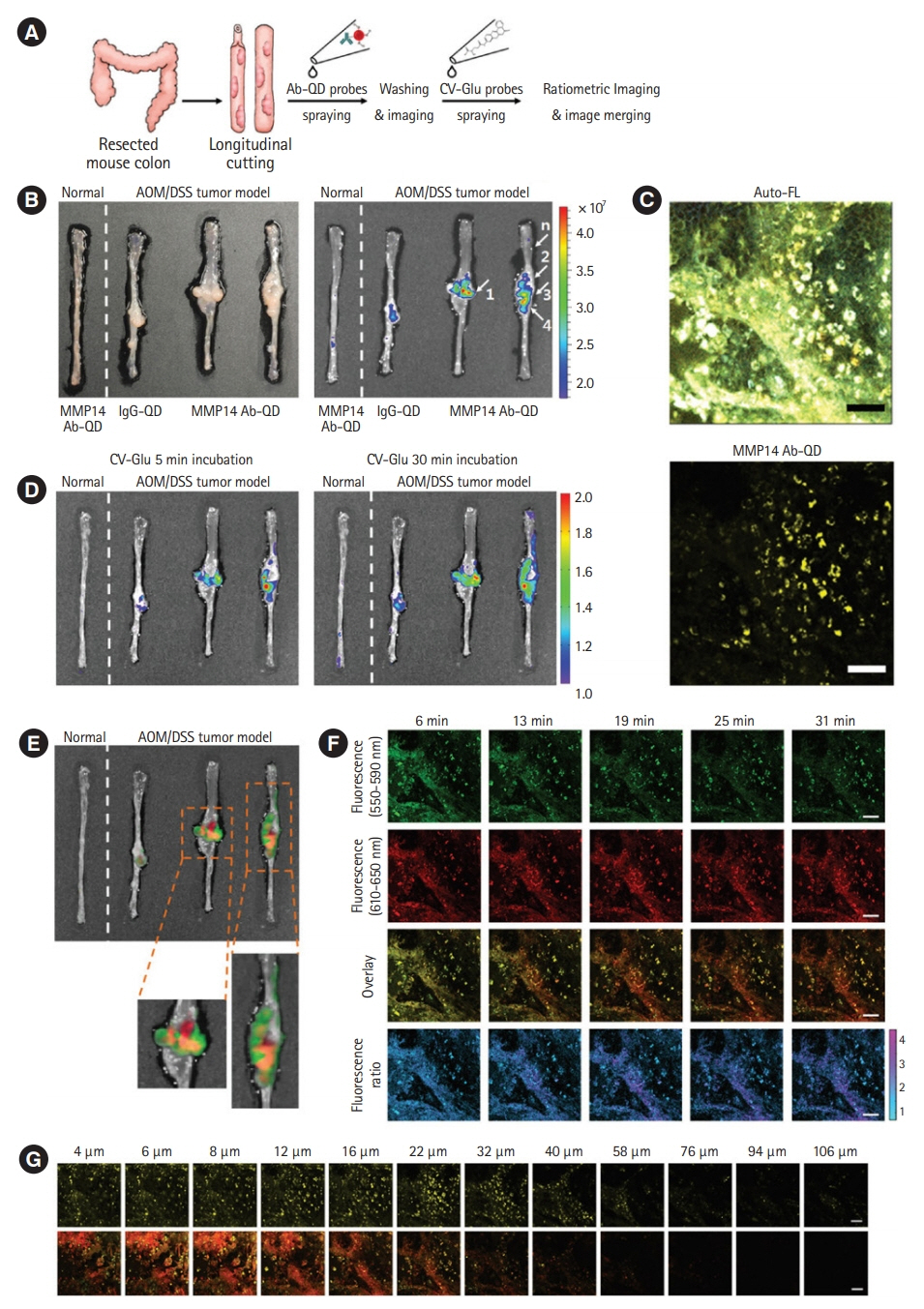
Article24. Park Y, Ryu YM, Jung Y, et al. Spraying quantum dot conjugates in the colon of live animals enabled rapid and multiplex cancer diagnosis using endoscopy. ACS Nano. 2014; 8:88968910.
Article25. Park S, Lim SY, Bae SM, Kim SY, Myung SJ, Kim HJ. Indocyanine-based activatable fluorescence turn-on probe for γ-glutamyltranspeptidase and its application to the mouse model of colon cancer. ACS Sens. 2016; 1:579–583.
Article26. Kim J, Do EJ, Moinova H, et al. Molecular imaging of colorectal tumors by targeting colon cancer secreted protein-2 (CCSP-2). Neoplasia. 2017; 19:805–816.
Article27. Bae SM, Park SJ, Choi M, et al. PSP1, a phosphatidylserinerecognizing peptide, is useful for visualizing radiation-induced apoptosis in colorectal cancer in vitro and in vivo. Transl Oncol. 2018; 11:1044–1052.
Article28. Park Y, Ryu YM, Wang T, et al. Colorectal cancer diagnosis using enzyme‐sensitive ratiometric fluorescence dye and antibody-quantum dot conjugates for multiplexed detection. Adv Funct Mater. 2018; 28:1703450.
Article29. Yang DH, Rey I. Endoscopic submucosal dissection for colitis-associated dysplasia. Clin Endosc. 2019; 52:120–128.
Article30. Jess T, Gamborg M, Matzen P, Munkholm P, Sørensen TI. Increased risk of intestinal cancer in Crohn’s disease: a metaanalysis of population-based cohort studies. Am J Gastroenterol. 2005; 100:2724–2729.
Article31. Jess T, Rungoe C, Peyrin-Biroulet L. Risk of colorectal cancer in patients with ulcerative colitis: a meta-analysis of population-based cohort studies. Clin Gastroenterol Hepatol. 2012; 10:639–645.
Article32. Kim BJ, Yang SK, Kim JS, et al. Trends of ulcerative colitis-associated colorectal cancer in Korea: a KASID study. J Gastroenterol Hepatol. 2009; 24:667–671.
Article33. Mattar MC, Lough D, Pishvaian MJ, Charabaty A. Current management of inflammatory bowel disease and colorectal cancer. Gastrointest Cancer Res. 2011; 4:53–61.34. Feagins LA, Souza RF, Spechler SJ. Carcinogenesis in IBD: potential targets for the prevention of colorectal cancer. Nat Rev Gastroenterol Hepatol. 2009; 6:297–305.
Article35. Rubin DC, Shaker A, Levin MS. Chronic intestinal inflammation: inflammatory bowel disease and colitis-associated colon cancer. Front Immunol. 2012; 3:107.
Article36. Saleh M, Trinchieri G. Innate immune mechanisms of colitis and colitis-associated colorectal cancer. Nat Rev Immunol. 2011; 11:9–20.
Article37. Kawanishi S, Hiraku Y, Pinlaor S, Ma N. Oxidative and nitrative DNA damage in animals and patients with inflammatory diseases in relation to inflammation-related carcinogenesis. Biol Chem. 2006; 387:365–372.
Article38. van Dieren JM, Wink JC, Vissers KJ, et al. Chromosomal and microsatellite instability of adenocarcinomas and dysplastic lesions (DALM) in ulcerative colitis. Diagn Mol Pathol. 2006; 15:216–222.
Article39. Tahara T, Inoue N, Hisamatsu T, et al. Clinical significance of microsatellite instability in the inflamed mucosa for the prediction of colonic neoplasms in patients with ulcerative colitis. J Gastroenterol Hepatol. 2005; 20:710–715.
Article40. Xie J, Itzkowitz SH. Cancer in inflammatory bowel disease. World J Gastroenterol. 2008; 14:378–389.
Article41. Itzkowitz S. Colon carcinogenesis in inflammatory bowel disease: applying molecular genetics to clinical practice. J Clin Gastroenterol. 2003; 36(5 Suppl):S70–S74.42. Itzkowitz SH, Harpaz N. Diagnosis and management of dysplasia in patients with inflammatory bowel diseases. Gastroenterology. 2004; 126:1634–1648.
Article43. Murthy SK, Kiesslich R. Evolving endoscopic strategies for detection and treatment of neoplastic lesions in inflammatory bowel disease. Gastrointest Endosc. 2013; 77:351–359.
Article44. Annese V, Beaugerie L, Egan L, et al. European evidencebased consensus: inflammatory bowel disease and malignancies. J Crohns Colitis. 2015; 9:945–965.
Article45. Farraye FA, Odze RD, Eaden J, Itzkowitz SH. AGA technical review on the diagnosis and management of colorectal neoplasia in inflammatory bowel disease. Gastroenterology. 2010; 138:746–774.
Article46. Cairns SR, Scholefield JH, Steele RJ, et al. Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups (update from 2002). Gut. 2010; 59:666689.
Article47. Kornbluth A, Sachar DB; Practice Parameters Committee of the American College of Gastroenterology. Ulcerative colitis practice guidelines in adults: American College of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. 2010; 105:501–523.
Article48. Awais D, Siegel CA, Higgins PD. Modelling dysplasia detection in ulcerative colitis: clinical implications of surveillance intensity. Gut. 2009; 58:1498–1503.
Article49. Subramanian V, Ramappa V, Telakis E, et al. Comparison of high definition with standard white light endoscopy for detection of dysplastic lesions during surveillance colonoscopy in patients with colonic inflammatory bowel disease. Inflamm Bowel Dis. 2013; 19:350–355.
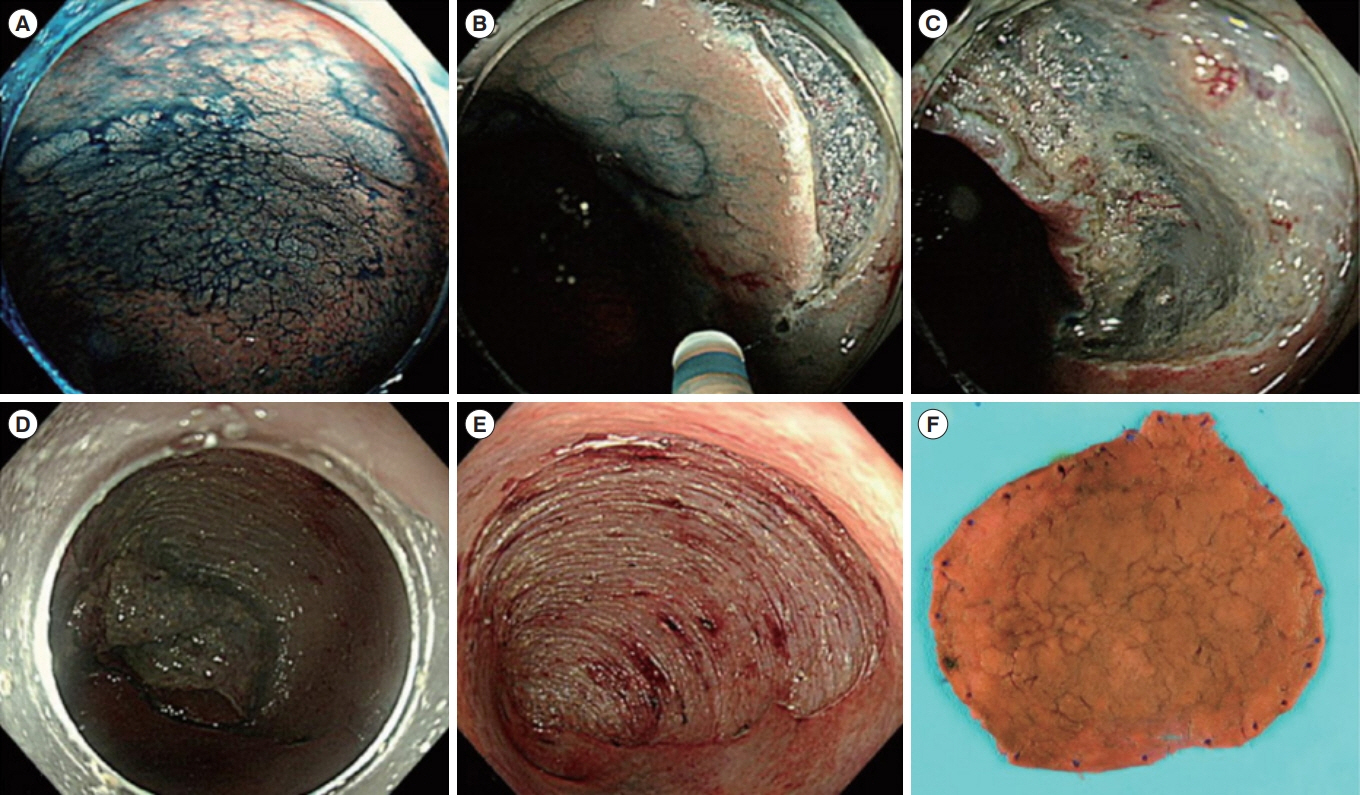
Article50. Kiesslich R, Neurath MF. Chromoendoscopy in inflammatory bowel disease. Gastroenterol Clin North Am. 2012; 41:291302.
Article51. Brown SR, Baraza W, Din S, Riley S. Chromoscopy versus conventional endoscopy for the detection of polyps in the colon and rectum. Cochrane Database Syst Rev. 2016; 4–CD006439.
Article52. Watanabe T, Ajioka Y, Mitsuyama K, et al. Comparison of targeted vs random biopsies for surveillance of ulcerative colitis-associated colorectal cancer. Gastroenterology. 2016; 151:1122–1130.
Article53. Rubin DT, Rothe JA, Hetzel JT, Cohen RD, Hanauer SB. Are dysplasia and colorectal cancer endoscopically visible in patients with ulcerative colitis? Gastrointest Endosc. 2007; 65:998–1004.
Article54. Rutter MD, Saunders BP, Wilkinson KH, Kamm MA, Williams CB, Forbes A. Most dysplasia in ulcerative colitis is visible at colonoscopy. Gastrointest Endosc. 2004; 60:334–339.
Article55. American Society for Gastrointestinal Endoscopy Standards of Practice Committee, Shergill AK, Lightdale JR, et al. The role of endoscopy in inflammatory bowel disease. Gastrointest Endosc. 2015; 81:1101–1121.
Article56. Laine L, Kaltenbach T, Barkun A, et al. SCENIC international consensus statement on surveillance and management of dysplasia in inflammatory bowel disease. Gastrointest Endosc. 2015; 81:489–501.
Article57. Velayos F, Kathpalia P, Finlayson E. Changing paradigms in detection of dysplasia and management of patients with inflammatory bowel disease: is colectomy still necessary? Gastroenterology. 2017; 152:440–450.
Article58. Annese V, Daperno M, Rutter MD, et al. European evidence based consensus for endoscopy in inflammatory bowel disease. J Crohns Colitis. 2013; 7:982–1018.
Article59. Iacopini F, Saito Y, Yamada M, et al. Curative endoscopic submucosal dissection of large nonpolypoid superficial neoplasms in ulcerative colitis (with videos). Gastrointest Endosc. 2015; 82:734–738.
Article60. Suzuki N, Toyonaga T, East JE. Endoscopic submucosal dissection of colitis-related dysplasia. Endoscopy. 2017; 49:12371242.
Article61. Yang DH, Kim J, Song EM, et al. Outcomes of ulcerative colitis-associated dysplasia patients referred for potential endoscopic submucosal dissection. J Gastroenterol Hepatol. 2019; 34:1581–1589.
Article62. Kinoshita S, Uraoka T, Nishizawa T, et al. The role of colorectal endoscopic submucosal dissection in patients with ulcerative colitis. Gastrointest Endosc. 2018; 87:1079–1084.
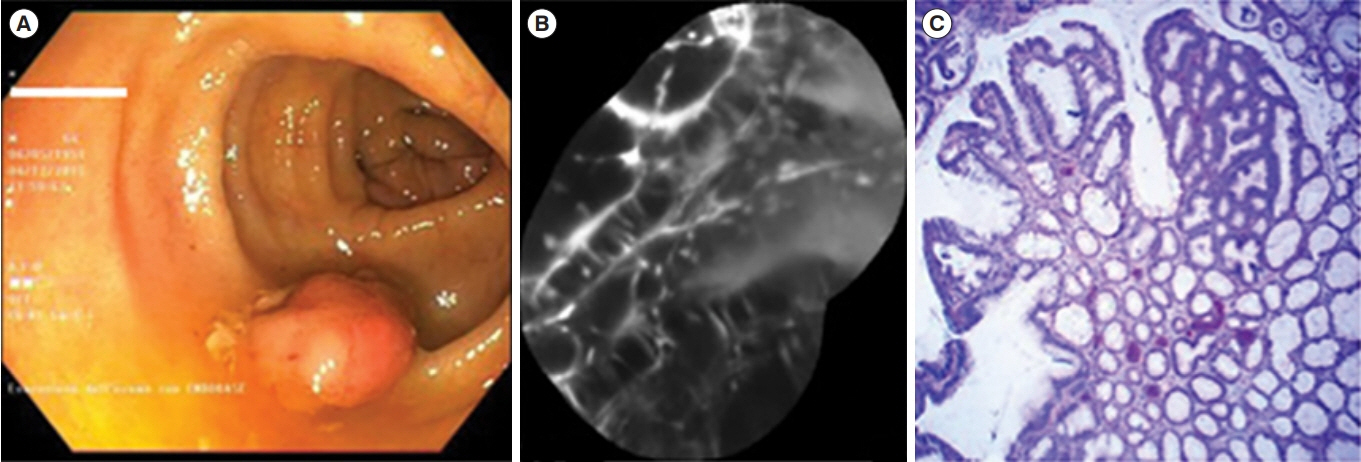
Article63. Gounaris E, Martin J, Ishihara Y, et al. Fluorescence endoscopy of cathepsin activity discriminates dysplasia from colitis. Inflamm Bowel Dis. 2013; 19:1339–1345.
Article64. Mason SD, Joyce JA. Proteolytic networks in cancer. Trends Cell Biol. 2011; 21:228–237.
Article65. Mitsunaga M, Kosaka N, Choyke PL, et al. Fluorescence endoscopic detection of murine colitis-associated colon cancer by topically applied enzymatically rapid-activatable probe. Gut. 2013; 62:1179–1186.
Article66. Pompella A, De Tata V, Paolicchi A, Zunino F. Expression of gamma-glutamyltransferase in cancer cells and its significance in drug resistance. Biochem Pharmacol. 2006; 71:231238.67. De Palma GD, Colavita I, Zambrano G, et al. Detection of colonic dysplasia in patients with ulcerative colitis using a targeted fluorescent peptide and confocal laser endomicroscopy: a pilot study. PLoS One. 2017; 12:e0180509.
Article68. Kiesslich R, Burg J, Vieth M, et al. Confocal laser endoscopy for diagnosing intraepithelial neoplasias and colorectal cancer in vivo. Gastroenterology. 2004; 127:706–713.
Article69. Kiesslich R, Goetz M, Lammersdorf K, et al. Chromoscopyguided endomicroscopy increases the diagnostic yield of intraepithelial neoplasia in ulcerative colitis. Gastroenterology. 2007; 132:874–882.
Article70. Caobelli F, Panarotto MB, Andreoli F, Ravelli A, De Agostini A, Giubbini R. Is 99mTc-HMPAO granulocyte scan an alternative to endoscopy in pediatric chronic inflammatory bowel disease (IBD)? Eur J Pediatr. 2011; 170:51–57.
Article71. Treglia G, Quartuccio N, Sadeghi R, et al. Diagnostic performance of fluorine-18-fluorodeoxyglucose positron emission tomography in patients with chronic inflammatory bowel disease: a systematic review and a meta-analysis. J Crohns Colitis. 2013; 7:345–354.
Article72. Caobelli F, Evangelista L, Quartuccio N, et al. Role of molecular imaging in the management of patients affected by inflammatory bowel disease: state-of-the-art. World J Radiol. 2016; 8:829–845.
Article73. Wang H, Machtaler S, Bettinger T, et al. Molecular imaging of inflammation in inflammatory bowel disease with a clinically translatable dual-selectin-targeted US contrast agent: comparison with FDG PET/CT in a mouse model. Radiology. 2013; 267:818–829.
Article74. Kiesslich R, Duckworth CA, Moussata D, et al. Local barrier dysfunction identified by confocal laser endomicroscopy predicts relapse in inflammatory bowel disease. Gut. 2012; 61:1146–1153.
Article75. Atreya R, Neumann H, Neufert C, et al. In vivo imaging using fluorescent antibodies to tumor necrosis factor predicts therapeutic response in Crohn’s disease. Nat Med. 2014; 20:313–318.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Advances in the Endoscopic Assessment of Inflammatory Bowel Diseases: Cooperation between Endoscopic and Pathologic Evaluations
- Role of Advanced Endoscopic Imaging Techniques in the Management of Inflammatory Bowel Disease
- Small Bowel Endoscopy in Inflammatory Bowel Disease
- Optical Molecular Imaging for Diagnosing Intestinal Diseases
- International Digestive Endoscopy Network to Strengthen Network for Lower Gastrointestinal Diseases Including Inflammatory Bowel Disease and Colorectal Cancer