Intest Res.
2021 Jan;19(1):1-11. 10.5217/ir.2020.00047.
Pouchitis in inflammatory bowel disease: a review of diagnosis, prognosis, and treatment
- Affiliations
-
- 1Inflammatory Bowel Disease Center, The University of Chicago Medicine, Chicago, IL, USA
- KMID: 2512096
- DOI: http://doi.org/10.5217/ir.2020.00047
Abstract
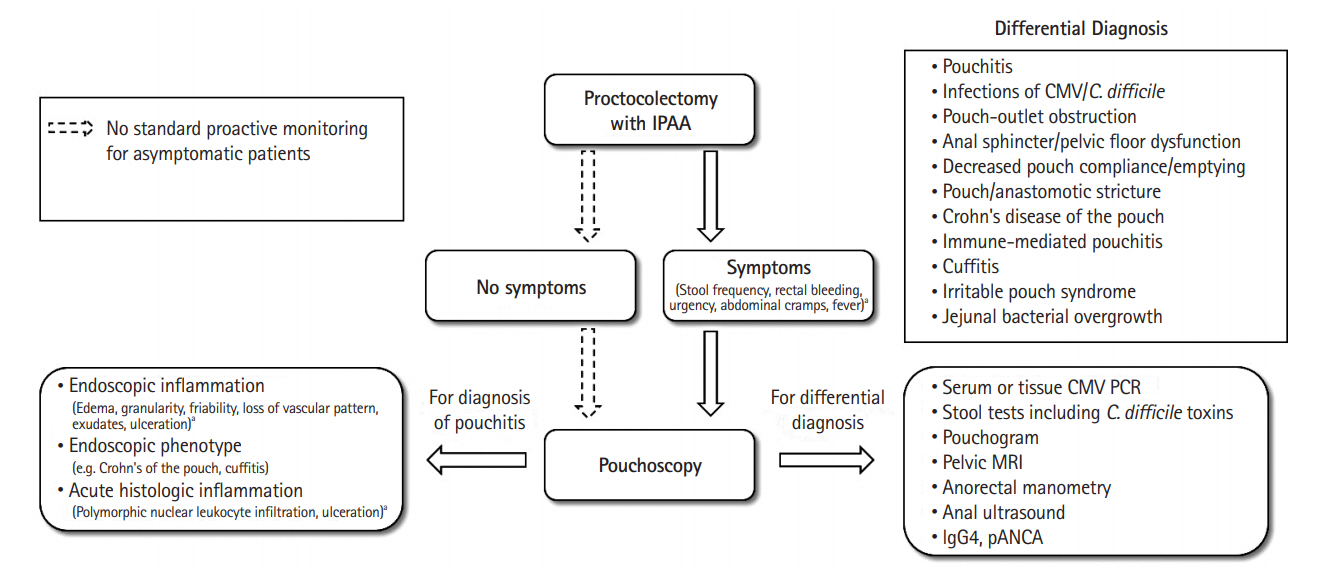
- Patients with inflammatory bowel disease (IBD) occasionally need a restorative proctocolectomy with ileal pouch-anal anastomosis (IPAA) because of medically refractory colitis or dysplasia/cancer. However, pouchitis may develop in up to 70% of patients after this procedure and significantly impair quality of life, more so if the inflammation becomes a chronic condition. About 10% of patients with IBD who develop pouchitis require pouch excision, and several risk factors of the failure have been reported. A phenotype that has features similar to Crohn’s disease may develop in a subset of ulcerative colitis patients following proctocolectomy with IPAA and is the most frequent reason for pouch failure. In this review, we discuss the diagnosis and prognosis of pouchitis, risk factors for pouchitis development, and treatment options for pouchitis, including the newer biological agents.
Figure
Cited by 1 articles
-
Association of colonic metaplasia of goblet cells and endoscopic phenotypes of the J pouch in patients with ulcerative colitis: a retrospective pilot study
Shintaro Akiyama, Tsubasa Onoda, Shoko Moue, Noriaki Sakamoto, Taku Sakamoto, Hideo Suzuki, Tsuyoshi Enomoto, Daisuke Matsubara, Tatsuya Oda, Kiichiro Tsuchiya
Intest Res. 2024;22(1):92-103. doi: 10.5217/ir.2023.00105.
Reference
-
1. Bernstein CN, Ng SC, Lakatos PL, Moum B, Loftus EV Jr; Epidemiology and Natural History Task Force of the International Organization of the Study of Inflammatory Bowel Disease. A review of mortality and surgery in ulcerative colitis: milestones of the seriousness of the disease. Inflamm Bowel Dis. 2013; 19:2001–2010.2. Abou Khalil M, Boutros M, Nedjar H, et al. Incidence rates and predictors of colectomy for ulcerative colitis in the era of biologics: results from a provincial database. J Gastrointest Surg. 2018; 22:124–132.
Article3. Fazio VW, Kiran RP, Remzi FH, et al. Ileal pouch anal anastomosis: analysis of outcome and quality of life in 3707 patients. Ann Surg. 2013; 257:679–685.4. Manilich E, Remzi FH, Fazio VW, Church JM, Kiran RP. Prognostic modeling of preoperative risk factors of pouch failure. Dis Colon Rectum. 2012; 55:393–399.
Article5. Tekkis PP, Lovegrove RE, Tilney HS, et al. Long-term failure and function after restorative proctocolectomy: a multi-centre study of patients from the UK National Ileal Pouch Registry. Colorectal Dis. 2010; 12:433–441.
Article6. Barnes EL, Kochar B, Jessup HR, Herfarth HH. The incidence and definition of Crohn’s disease of the pouch: a systematic review and meta-analysis. Inflamm Bowel Dis. 2019; 25:1474–1480.
Article7. Hata K, Ishihara S, Nozawa H, et al. Pouchitis after ileal pouchanal anastomosis in ulcerative colitis: diagnosis, management, risk factors, and incidence. Dig Endosc. 2017; 29:26–34.
Article8. Sandborn WJ. Pouchitis following ileal pouch-anal anastomosis: definition, pathogenesis, and treatment. Gastroenterology. 1994; 107:1856–1860.
Article9. Sandborn WJ, Tremaine WJ, Batts KP, Pemberton JH, Phillips SF. Pouchitis after ileal pouch-anal anastomosis: a pouchitis disease activity index. Mayo Clin Proc. 1994; 69:409–415.
Article10. Shen B, Achkar JP, Connor JT, et al. Modified pouchitis disease activity index: a simplified approach to the diagnosis of pouchitis. Dis Colon Rectum. 2003; 46:748–753.11. Mignon M, Stettler C, Phillips SF. Pouchitis: a poorly understood entity. Dis Colon Rectum. 1995; 38:100–103.12. Pardi DS, Sandborn WJ. Systematic review: the management of pouchitis. Aliment Pharmacol Ther. 2006; 23:1087–1096.
Article13. Seril DN, Yao Q, Shen B. The association between autoimmunity and pouchitis. Inflamm Bowel Dis. 2014; 20:378–388.
Article14. Shen B, Achkar JP, Lashner BA, et al. Endoscopic and histologic evaluation together with symptom assessment are required to diagnose pouchitis. Gastroenterology. 2001; 121:261–267.
Article15. Shen B, Achkar JP, Lashner BA, et al. Irritable pouch syndrome: a new category of diagnosis for symptomatic patients with ileal pouch-anal anastomosis. Am J Gastroenterol. 2002; 97:972–977.
Article16. O’Connell PR, Rankin DR, Weiland LH, Kelly KA. Enteric bacteriology, absorption, morphology and emptying after ileal pouch-anal anastomosis. Br J Surg. 1986; 73:909–914.
Article17. Zhu H, Wu XR, Queener E, Kiran RP, Remzi FH, Shen B. Clinical value of surveillance pouchoscopy in asymptomatic ileal pouch patients with underlying inflammatory bowel disease. Surg Endosc. 2013; 27:4325–4332.
Article18. Kayal M, Plietz M, Radcliffe M, et al. Endoscopic activity in asymptomatic patients with an ileal pouch is associated with an increased risk of pouchitis. Aliment Pharmacol Ther. 2019; 50:1189–1194.
Article19. Khan F, Shen B. Inflammation and neoplasia of the pouch in inflammatory bowel disease. Curr Gastroenterol Rep. 2019; 21:10.
Article20. Sahami S, Bartels SA, D’Hoore A, et al. External validation of a prognostic model of preoperative risk factors for failure of restorative proctocolectomy. Colorectal Dis. 2017; 19:181–187.
Article21. de Zeeuw S, Ahmed Ali U, Donders RA, Hueting WE, Keus F, van Laarhoven CJ. Update of complications and functional outcome of the ileo-pouch anal anastomosis: overview of evidence and meta-analysis of 96 observational studies. Int J Colorectal Dis. 2012; 27:843–853.
Article22. Ide S, Araki T, Okita Y, et al. Outcome and functional prognosis of pelvic sepsis after ileal pouch-anal anastomosis in patients with ulcerative colitis. Surg Today. 2017; 47:301–306.
Article23. Fazio VW, Tekkis PP, Remzi F, et al. Quantification of risk for pouch failure after ileal pouch anal anastomosis surgery. Ann Surg. 2003; 238:605–614.
Article24. Cohen Z, McLeod RS, Stephen W, Stern HS, O’Connor B, Reznick R. Continuing evolution of the pelvic pouch procedure. Ann Surg. 1992; 216:506–511.
Article25. Ziv Y, Fazio VW, Church JM, Lavery IC, King TM, Ambrosetti P. Stapled ileal pouch anal anastomoses are safer than handsewn anastomoses in patients with ulcerative colitis. Am J Surg. 1996; 171:320–323.
Article26. Lovegrove RE, Constantinides VA, Heriot AG, et al. A comparison of hand-sewn versus stapled ileal pouch anal anastomosis (IPAA) following proctocolectomy: a meta-analysis of 4183 patients. Ann Surg. 2006; 244:18–26.
Article27. Lightner AL, Pemberton JH, Loftus EJ Jr. Crohn’s disease of the ileoanal pouch. Inflamm Bowel Dis. 2016; 22:1502–1508.
Article28. Shen B, Remzi FH, Brzezinski A, et al. Risk factors for pouch failure in patients with different phenotypes of Crohn’s disease of the pouch. Inflamm Bowel Dis. 2008; 14:942–948.
Article29. Pellino G, Vinci D, Signoriello G, et al. Long-term bowel function and fate of the ileal pouch after restorative proctocolectomy in patients with Crohn’s disease: a systematic review with meta-analysis and metaregression. J Crohns Colitis. 2020; 14:418–427.
Article30. Magill SS, Edwards JR, Bamberg W, et al. Multistate pointprevalence survey of health care-associated infections. N Engl J Med. 2014; 370:1198–1208.
Article31. Razik R, Rumman A, Bahreini Z, McGeer A, Nguyen GC. Recurrence of Clostridium difficile infection in patients with inflammatory bowel disease: the recidivism study. Am J Gastroenterol. 2016; 111:1141–1146.
Article32. Lightner AL, Tse CS, Quinn K, et al. Preoperative Clostridium difficile infection does not affect pouch outcomes in patients with ulcerative colitis who undergo ileal pouch-anal anastomosis. Inflamm Bowel Dis. 2017; 23:1195–1201.
Article33. Skowron KB, Lapin B, Rubin M, et al. Clostridium difficile infection in ulcerative colitis: can alteration of the gut-associated microbiome contribute to pouch failure? Inflamm Bowel Dis. 2016; 22:902–911.34. Hata K, Ishii H, Anzai H, et al. Preoperative extraintestinal manifestations associated with chronic pouchitis in Japanese patients with ulcerative colitis after ileal pouch-anal anastomosis: a retrospective study. Inflamm Bowel Dis. 2017; 23:1019–1024.
Article35. Fleshner P, Ippoliti A, Dubinsky M, et al. A prospective multivariate analysis of clinical factors associated with pouchitis after ileal pouch-anal anastomosis. Clin Gastroenterol Hepatol. 2007; 5:952–958.
Article36. LaRusso NF, Wiesner RH, Ludwig J, MacCarty RL. Current concepts. Primary sclerosing cholangitis. N Engl J Med. 1984; 310:899–903.37. Loftus EV Jr, Sandborn WJ, Lindor KD, Larusso NF. Interactions between chronic liver disease and inflammatory bowel disease. Inflamm Bowel Dis. 1997; 3:288–302.
Article38. Loftus EV Jr, Harewood GC, Loftus CG, et al. PSC-IBD: a unique form of inflammatory bowel disease associated with primary sclerosing cholangitis. Gut. 2005; 54:91–96.
Article39. Abdelrazeq AS, Kandiyil N, Botterill ID, et al. Predictors for acute and chronic pouchitis following restorative proctocolectomy for ulcerative colitis. Colorectal Dis. 2008; 10:805–813.
Article40. Pavlides M, Cleland J, Rahman M, et al. Outcomes after ileal pouch anal anastomosis in patients with primary sclerosing cholangitis. J Crohns Colitis. 2014; 8:662–670.
Article41. Penna C, Dozois R, Tremaine W, et al. Pouchitis after ileal pouch-anal anastomosis for ulcerative colitis occurs with increased frequency in patients with associated primary sclerosing cholangitis. Gut. 1996; 38:234–239.
Article42. Tenca A, Jaakkola T, Färkkilä M, Arola J, Kolho KL. Impact of paediatric onset primary sclerosing cholangitis on clinical course and outcome of inflammatory bowel disease: a casecontrol population-based study in Finland. Scand J Gastroenterol. 2019; 54:984–990.
Article43. White E, Melmed GY, Vasiliauskas EA, et al. A prospective analysis of clinical variables, serologic factors, and outcome of ileal pouch-anal anastomosis in patients with backwash ileitis. Dis Colon Rectum. 2010; 53:987–994.
Article44. Hashavia E, Dotan I, Rabau M, Klausner JM, Halpern Z, Tulchinsky H. Risk factors for chronic pouchitis after ileal pouch-anal anastomosis: a prospective cohort study. Colorectal Dis. 2012; 14:1365–1371.
Article45. Merrett MN, Mortensen N, Kettlewell M, Jewell DO. Smoking may prevent pouchitis in patients with restorative proctocolectomy for ulcerative colitis. Gut. 1996; 38:362–364.
Article46. Shen B, Fazio VW, Remzi FH, et al. Risk factors for diseases of ileal pouch-anal anastomosis after restorative proctocolectomy for ulcerative colitis. Clin Gastroenterol Hepatol. 2006; 4:81–89.
Article47. Gorrepati VS, Stuart A, Deiling S, et al. Smoking and the risk of pouchitis in ulcerative colitis patients with ileal pouch-anal anastomosis. Inflamm Bowel Dis. 2018; 24:2027–2032.
Article48. To N, Ford AC, Gracie DJ. Systematic review with meta-analysis: the effect of tobacco smoking on the natural history of ulcerative colitis. Aliment Pharmacol Ther. 2016; 44:117–126.
Article49. Achkar JP, Al-Haddad M, Lashner B, et al. Differentiating risk factors for acute and chronic pouchitis. Clin Gastroenterol Hepatol. 2005; 3:60–66.
Article50. Wu XR, Ashburn J, Remzi FH, Li Y, Fass H, Shen B. Male gender is associated with a high risk for chronic antibiotic-refractory pouchitis and ileal pouch anastomotic sinus. J Gastrointest Surg. 2016; 20:631–639.
Article51. Tiainen J, Matikainen M. Long-term clinical outcome and anemia after restorative proctocolectomy for ulcerative colitis. Scand J Gastroenterol. 2000; 35:1170–1173.
Article52. Singh S, Sharma PK, Loftus EV Jr, Pardi DS. Meta-analysis: serological markers and the risk of acute and chronic pouchitis. Aliment Pharmacol Ther. 2013; 37:867–875.
Article53. Araki T, Hashimoto K, Okita Y, et al. Colonic histological criteria predict development of pouchitis after ileal pouch: anal anastomosis for patients with ulcerative colitis. Dig Surg. 2018; 35:138–143.
Article54. Dipasquale V, Mattioli G, Arrigo S, et al. Pouchitis in pediatric ulcerative colitis: a multicenter study on behalf of Italian Society of Pediatric Gastroenterology, Hepatology and Nutrition. Dig Liver Dis. 2019; 51:1551–1556.
Article55. Hurst RD, Molinari M, Chung TP, Rubin M, Michelassi F. Prospective study of the incidence, timing and treatment of pouchitis in 104 consecutive patients after restorative proctocolectomy. Arch Surg. 1996; 131:497–500.
Article56. Isaacs KL, Sandler RS, Abreu M, et al. Rifaximin for the treatment of active pouchitis: a randomized, double-blind, placebo-controlled pilot study. Inflamm Bowel Dis. 2007; 13:1250–1255.
Article57. Magro F, Gionchetti P, Eliakim R, et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 1: definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and ileo-anal pouch disorders. J Crohns Colitis. 2017; 11:649–670.
Article58. Shen B, Achkar JP, Lashner BA, et al. A randomized clinical trial of ciprofloxacin and metronidazole to treat acute pouchitis. Inflamm Bowel Dis. 2001; 7:301–305.
Article59. Gionchetti P, Rizzello F, Helwig U, et al. Prophylaxis of pouchitis onset with probiotic therapy: a double-blind, placebo-controlled trial. Gastroenterology. 2003; 124:1202–1209.
Article60. Mimura T, Rizzello F, Helwig U, et al. Four-week open-label trial of metronidazole and ciprofloxacin for the treatment of recurrent or refractory pouchitis. Aliment Pharmacol Ther. 2002; 16:909–917.61. Abdelrazeq AS, Kelly SM, Lund JN, Leveson SH. Rifaximinciprofloxacin combination therapy is effective in chronic active refractory pouchitis. Colorectal Dis. 2005; 7:182–186.
Article62. Gionchetti P, Rizzello F, Poggioli G, et al. Oral budesonide in the treatment of chronic refractory pouchitis. Aliment Pharmacol Ther. 2007; 25:1231–1236.
Article63. Uchino M, Ikeuchi H, Matsuoka H, et al. Topical tacrolimus therapy for antibiotic-refractory pouchitis. Dis Colon Rectum. 2013; 56:1166–1173.
Article64. Gionchetti P, Calabrese C, Calafiore A, et al. Oral beclomethasone dipropionate in chronic refractory pouchitis. J Crohns Colitis. 2014; 8:649–653.
Article65. Mimura T, Rizzello F, Helwig U, et al. Once daily high dose probiotic therapy (VSL#3) for maintaining remission in recurrent or refractory pouchitis. Gut. 2004; 53:108–114.
Article66. Gionchetti P, Rizzello F, Venturi A, et al. Oral bacteriotherapy as maintenance treatment in patients with chronic pouchitis: a double-blind, placebo-controlled trial. Gastroenterology. 2000; 119:305–309.
Article67. Liu Z, Song H, Shen B. Pouchitis: prevention and treatment. Curr Opin Clin Nutr Metab Care. 2014; 17:489–495.68. Ferrante M, D’Haens G, Dewit O, et al. Efficacy of infliximab in refractory pouchitis and Crohn’s disease-related complications of the pouch: a Belgian case series. Inflamm Bowel Dis. 2010; 16:243–249.
Article69. Barreiro-de Acosta M, García-Bosch O, Souto R, et al. Efficacy of infliximab rescue therapy in patients with chronic refractory pouchitis: a multicenter study. Inflamm Bowel Dis. 2012; 18:812–817.
Article70. Kelly OB, Rosenberg M, Tyler AD, et al. Infliximab to treat refractory inflammation after pelvic pouch surgery for ulcerative colitis. J Crohns Colitis. 2016; 10:410–417.
Article71. Uchino M, Ikeuchi H, Bando T, et al. Clinical features of refractory pouchitis with penetrating lesions and the efficacy of infliximab treatment for patients with ulcerative colitis after restorative proctocolectomy. Digestion. 2015; 92:147–155.
Article72. Barreiro-de Acosta M, García-Bosch O, Gordillo J, et al. Efficacy of adalimumab rescue therapy in patients with chronic refractory pouchitis previously treated with infliximab: a case series. Eur J Gastroenterol Hepatol. 2012; 24:756–758.
Article73. Kjær MD, Qvist N, Nordgaard-Lassen I, Christensen LA, Kjeldsen J. Adalimumab in the treatment of chronic pouchitis: a randomized double-blind, placebo-controlled trial. Scand J Gastroenterol. 2019; 54:188–193.
Article74. Segal JP, Ding NS, Worley G, et al. Systematic review with meta-analysis: the management of chronic refractory pouchitis with an evidence-based treatment algorithm. Aliment Pharmacol Ther. 2017; 45:581–592.75. Herfarth HH, Long MD, Isaacs KL. Use of biologics in pouchitis: a systematic review. J Clin Gastroenterol. 2015; 49:647–654.76. Huguet M, Pereira B, Goutte M, et al. Systematic review with meta-analysis: anti-TNF therapy in refractory pouchitis and Crohn’s disease-like complications of the pouch after ileal pouch-anal anastomosis following colectomy for ulcerative colitis. Inflamm Bowel Dis. 2018; 24:261–268.
Article77. Fedyk ER, Wyant T, Yang LL, et al. Exclusive antagonism of the α4β7 integrin by vedolizumab confirms the gut-selectivity of this pathway in primates. Inflamm Bowel Dis. 2012; 18:2107–2119.
Article78. Bär F, Kühbacher T, Dietrich NA, et al. Vedolizumab in the treatment of chronic, antibiotic-dependent or refractory pouchitis. Aliment Pharmacol Ther. 2018; 47:581–587.
Article79. Gregory M, Weaver KN, Hoversten P, et al. Efficacy of vedolizumab for refractory pouchitis of the ileo-anal pouch: results from a multicenter US cohort. Inflamm Bowel Dis. 2019; 25:1569–1576.80. Sandborn WJ, Gasink C, Gao LL, et al. Ustekinumab induction and maintenance therapy in refractory Crohn’s disease. N Engl J Med. 2012; 367:1519–1528.
Article81. Sands BE, Sandborn WJ, Panaccione R, et al. Ustekinumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2019; 381:1201–1214.
Article82. Ollech JE, Rubin DT, Glick L, et al. Ustekinumab is effective for the treatment of chronic antibiotic-refractory pouchitis. Dig Dis Sci. 2019; 64:3596–3601.
Article83. Colombel JF, Ricart E, Loftus EV Jr, et al. Management of Crohn’s disease of the ileoanal pouch with infliximab. Am J Gastroenterol. 2003; 98:2239–2244.
Article84. Shah H, Zezos P. Pouchitis: diagnosis and management. Curr Opin Gastroenterol. 2020; 36:41–47.85. Weaver KN, Gregory M, Syal G, et al. Ustekinumab is effective for the treatment of Crohn’s disease of the pouch in a multicenter cohort. Inflamm Bowel Dis. 2019; 25:767–774.
Article86. Ardalan ZS, Sparrow M, Gibson PR. The importance of accurate phenotyping and pouchitis risk and dietary assessment when investigating the microbial factors behind antibiotic-dependent pouchitis. Gastroenterology. 2020; 159:399–400.
Article