Nutr Res Pract.
2021 Feb;15(1):1-11. 10.4162/nrp.2021.15.1.1.
Validation of soy isoflavone intake and its health effects: a review of the development of exposure biomarkers
- Affiliations
-
- 1National Institute of Agricultural Sciences, Rural Development Administration, Wanju 55365, Korea
- 2Department of Nutritional Science and Food Management, Ewha Womans University, Seoul 03765, Korea
- 3Division of Applied Food System, Major of Food and Nutrition, Seoul Women's University, Seoul 01797, Korea
- KMID: 2511670
- DOI: http://doi.org/10.4162/nrp.2021.15.1.1
Abstract
- BACKGROUND/OBJECTIVES
It is difficult to consistently demonstrate the health effects of soy isoflavones owing to the multitude of factors contributing to their bioavailability. To accurately verify these health effects, dietary isoflavone intake should be measured using a biologically active dose rather than an intake dose. This concept has been expanded to the development of new exposure biomarkers in nutrition research. This review aims to provide an overview of the development of exposure biomarkers and suggest a novel research strategy for identifying the health effects of soy isoflavone intake.
MATERIALS/METHODS
We cover recent studies on the health effects of soy isoflavones focusing on isoflavone metabolites as exposure biomarkers.
RESULTS
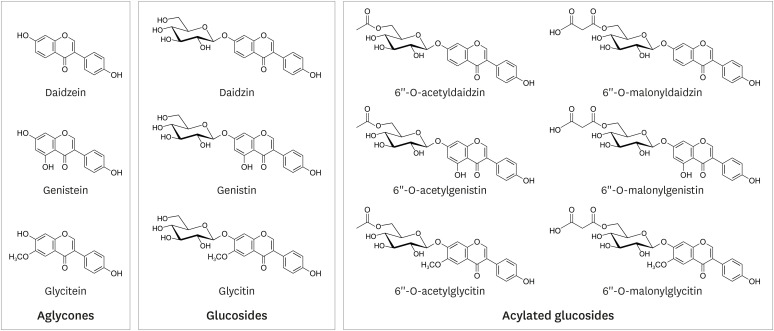
Compared to non-fermented soy foods, fermented soy foods cause an increased concentration of isoflavones in the biofluid immediately following ingestion. The correlation between exposure biomarkers in blood and urine and the food frequency questionnaire was slightly lower than that of corresponding 24-h dietary recalls. Urinary and blood isoflavone levels did not show a consistent association with chronic disease and cancer risk.
CONCLUSION
It is crucial to understand the variable bioavailabilities of soy isoflavones, which may affect evaluations of soy isoflavone intake in health and disease. Further studies on the development of valid exposure biomarkers are needed to thoroughly investigate the health effects of isoflavone.
Keyword
Figure
Reference
-
1. Praticò G, Gao Q, Scalbert A, Vergères G, Kolehmainen M, Manach C, Brennan L, Pedapati SH, Afman LA, Wishart DS, Vázquez-Fresno R, Andres-Lacueva C, Garcia-Aloy M, Verhagen H, Feskens EJ, Dragsted LO. Guidelines for Biomarker of Food Intake Reviews (BFIRev): how to conduct an extensive literature search for biomarker of food intake discovery. Genes Nutr. 2018; 13:3. PMID: 29484030.
Article2. Kwon O. A big picture view of precision nutrition: from reductionism to holism. J Nutr Health. 2019; 52:1–5.
Article3. Gao Q, Praticò G, Scalbert A, Vergères G, Kolehmainen M, Manach C, Brennan L, Afman LA, Wishart DS, Andres-Lacueva C, Garcia-Aloy M, Verhagen H, Feskens EJ, Dragsted LO. A scheme for a flexible classification of dietary and health biomarkers. Genes Nutr. 2017; 12:34. PMID: 29255495.
Article4. Shi L, Brunius C, Bergdahl IA, Johansson I, Rolandsson O, Donat Vargas C, Kiviranta H, Hanhineva K, Åkesson A, Landberg R. Joint analysis of metabolite markers of fish intake and persistent organic pollutants in relation to type 2 diabetes risk in Swedish adults. J Nutr. 2019; 149:1413–1423. PMID: 31209490.
Article5. Loftfield E, Rothwell JA, Sinha R, Keski-Rahkonen P, Robinot N, Albanes D, Weinstein SJ, Derkach A, Sampson J, Scalbert A, Freedman ND. Prospective investigation of serum metabolites, coffee drinking, liver cancer incidence, and liver disease mortality. J Natl Cancer Inst. 2020; 112:286–294. PMID: 31168595.
Article6. Lampe JW. Isoflavonoid and lignan phytoestrogens as dietary biomarkers. J Nutr. 2003; 133(Suppl 3):956S–964S. PMID: 12612182.
Article7. Holst B, Williamson G. Nutrients and phytochemicals: from bioavailability to bioefficacy beyond antioxidants. Curr Opin Biotechnol. 2008; 19:73–82. PMID: 18406129.
Article8. Maruvada P, Lampe JW, Wishart DS, Barupal D, Chester DN, Dodd D, Djoumbou-Feunang Y, Dorrestein PC, Dragsted LO, Draper J, Duffy LC, Dwyer JT, Emenaker NJ, Fiehn O, Gerszten RE, Hu FB, Karp RW, Klurfeld DM, Laughlin MR, Little AR, Lynch CJ, Moore SC, Nicastro HL, O'Brien DM, Ordovás JM, Osganian SK, Playdon M, Prentice R, Raftery D, Reisdorph N, Roche HM, Ross SA, Sang S, Scalbert A, Srinivas PR, Zeisel SH. Perspective: dietaryiomarkers of intake and exposure-exploration with omics approaches. Adv Nutr. 2020; 11:200–215. PMID: 31386148.9. Heinzmann SS, Holmes E, Kochhar S, Nicholson JK, Schmitt-Kopplin P. Correction to 2-Furoylglycine as a Candidate Biomarker of Coffee Consumption. J Agric Food Chem. 2016; 64:8958. PMID: 27934290.
Article10. Cheung W, Keski-Rahkonen P, Assi N, Ferrari P, Freisling H, Rinaldi S, Slimani N, Zamora-Ros R, Rundle M, Frost G, Gibbons H, Carr E, Brennan L, Cross AJ, Pala V, Panico S, Sacerdote C, Palli D, Tumino R, Kühn T, Kaaks R, Boeing H, Floegel A, Mancini F, Boutron-Ruault MC, Baglietto L, Trichopoulou A, Naska A, Orfanos P, Scalbert A. A metabolomic study of biomarkers of meat and fish intake. Am J Clin Nutr. 2017; 105:600–608. PMID: 28122782.
Article11. Andersen MB, Reinbach HC, Rinnan Å, Barri T, Mithril C, Dragsted LO. Discovery of exposure markers in urine for Brassica-containing meals served with different protein sources by UPLC-qTOF-MS untargeted metabolomics. Metabolomics. 2013; 9:984–997.12. Urpi‐Sarda M, Boto‐Ordóñez M, Queipo‐Ortuño MI, Tulipani S, Corella D, Estruch R, Tinahones FJ, Andres‐Lacueva C. Phenolic and microbial‐targeted metabolomics to discovering and evaluating wine intake biomarkers in human urine and plasma. Electrophoresis. 2015; 36:2259–2268. PMID: 25929678.
Article13. Mitry P, Wawro N, Rohrmann S, Giesbertz P, Daniel H, Linseisen J. Plasma concentrations of anserine, carnosine and pi-methylhistidine as biomarkers of habitual meat consumption. Eur J Clin Nutr. 2019; 73:692–702. PMID: 30018457.
Article14. Garcia-Aloy M, Llorach R, Urpi-Sarda M, Tulipani S, Salas-Salvadó J, Martínez-González MA, Corella D, Fitó M, Estruch R, Serra-Majem L, Andres-Lacueva C. Nutrimetabolomics fingerprinting to identify biomarkers of bread exposure in a free-living population from the PREDIMED study cohort. Metabolomics. 2015; 11:155–165.
Article15. Zhu Y, Wang P, Sha W, Sang S. Urinary biomarkers of whole grain wheat intake identified by non-targeted and targeted metabolomics approaches. Sci Rep. 2016; 6:36278. PMID: 27805021.
Article16. Miles FL, Lloren JI, Haddad E, Jaceldo-Siegl K, Knutsen S, Sabate J, Fraser GE. Plasma, urine, and adipose tissue biomarkers of dietary intake differ between vegetarian and non-vegetarian diet groups in the Adventist Health Study-2. J Nutr. 2019; 149:667–675. PMID: 30770530.
Article17. Akbaraly T, Würtz P, Singh-Manoux A, Shipley MJ, Haapakoski R, Lehto M, Desrumaux C, Kähönen M, Lehtimäki T, Mikkilä V, Hingorani A, Humphries SE, Kangas AJ, Soininen P, Raitakari O, Ala-Korpela M, Kivimäki M. Association of circulating metabolites with healthy diet and risk of cardiovascular disease: analysis of two cohort studies. Sci Rep. 2018; 8:8620. PMID: 29872056.
Article18. Setchell KD. Absorption and metabolism of soy isoflavones-from food to dietary supplements and adults to infants. J Nutr. 2000; 130:654S–655S. PMID: 10702601.
Article19. de Pascual-Teresa S, Hallund J, Talbot D, Schroot J, Williams CM, Bugel S, Cassidy A. Absorption of isoflavones in humans: effects of food matrix and processing. J Nutr Biochem. 2006; 17:257–264. PMID: 16109484.
Article20. Zhang Y, Hendrich S, Murphy PA. Glucuronides are the main isoflavone metabolites in women. J Nutr. 2003; 133:399–404. PMID: 12566474.
Article21. Nielsen IL, Williamson G. Review of the factors affecting bioavailability of soy isoflavones in humans. Nutr Cancer. 2007; 57:1–10. PMID: 17516857.
Article22. Setchell KD. The history and basic science development of soy isoflavones. Menopause. 2017; 24:1338–1350. PMID: 29189602.
Article23. Zeng M, Sun R, Basu S, Ma Y, Ge S, Yin T, Gao S, Zhang J, Hu M. Disposition of flavonoids via recycling: Direct biliary excretion of enterically or extrahepatically derived flavonoid glucuronides. Mol Nutr Food Res. 2016; 60:1006–1019. PMID: 26843117.
Article24. Barnes S. The biochemistry, chemistry and physiology of the isoflavones in soybeans and their food products. Lymphat Res Biol. 2010; 8:89–98. PMID: 20235891.
Article25. Lee DH, Kim MJ, Ahn J, Lee SH, Lee H, Kim JH, Park SH, Jang YJ, Ha TY, Jung CH. Nutrikinetics of isoflavone metabolites after fermented soybean product (cheonggukjang) ingestion in ovariectomized mice. Mol Nutr Food Res. 2017; 61:1700322.
Article26. Shelnutt SR, Cimino CO, Wiggins PA, Ronis MJ, Badger TM. Pharmacokinetics of the glucuronide and sulfate conjugates of genistein and daidzein in men and women after consumption of a soy beverage. Am J Clin Nutr. 2002; 76:588–594. PMID: 12198004.
Article27. Ronis MJ, Little JM, Barone GW, Chen G, Radominska-Pandya A, Badger TM. Sulfation of the isoflavones genistein and daidzein in human and rat liver and gastrointestinal tract. J Med Food. 2006; 9:348–355. PMID: 17004897.
Article28. D'Archivio M, Filesi C, Varì R, Scazzocchio B, Masella R. Bioavailability of the polyphenols: status and controversies. Int J Mol Sci. 2010; 11:1321–1342. PMID: 20480022.29. Yonemoto-Yano H, Maebuchi M, Fukui K, Tsuzaki S, Takamatsu K, Uehara M. Malonyl isoflavone glucosides are chiefly hydrolyzed and absorbed in the colon. J Agric Food Chem. 2014; 62:2264–2270. PMID: 24524651.
Article30. Okabe Y, Shimazu T, Tanimoto H. Higher bioavailability of isoflavones after a single ingestion of aglycone-rich fermented soybeans compared with glucoside-rich non-fermented soybeans in Japanese postmenopausal women. J Sci Food Agric. 2011; 91:658–663. PMID: 21104834.
Article31. Kano M, Takayanagi T, Harada K, Sawada S, Ishikawa F. Bioavailability of isoflavones after ingestion of soy beverages in healthy adults. J Nutr. 2006; 136:2291–2296. PMID: 16920843.
Article32. Tsangalis D, Wilcox G, Shah NP, Stojanovska L. Bioavailability of isoflavone phytoestrogens in postmenopausal women consuming soya milk fermented with probiotic bifidobacteria. Br J Nutr. 2005; 93:867–877. PMID: 16022756.
Article33. Rienks J, Barbaresko J, Nöthlings U. Association of isoflavone biomarkers with risk of chronic disease and mortality: a systematic review and meta-analysis of observational studies. Nutr Rev. 2017; 75:616–641. PMID: 28969363.
Article34. Kim J, Kim HJ, Joung H, Park MK, Li S, Song Y, Franke AA, Paik HY. Overnight urinary excretion of isoflavones as an indicator for dietary isoflavone intake in Korean girls of pubertal age. Br J Nutr. 2010; 104:709–715. PMID: 20385037.
Article35. Morimoto Y, Beckford F, Franke AA, Maskarinec G. Urinary isoflavonoid excretion as a biomarker of dietary soy intake during two randomized soy trials. Asia Pac J Clin Nutr. 2014; 23:205–209. PMID: 24901088.36. Atkinson C, Skor HE, Fitzgibbons ED, Scholes D, Chen C, Wähälä K, Schwartz SM, Lampe JW. Overnight urinary isoflavone excretion in a population of women living in the United States, and its relationship to isoflavone intake. Cancer Epidemiol Biomarkers Prev. 2002; 11:253–260. PMID: 11895874.37. Chávez-Suárez KM, Ortega-Vélez MI, Valenzuela-Quintanar AI, Galván-Portillo M, López-Carrillo L, Esparza-Romero J, Saucedo-Tamayo MS, Robles-Burgueño MR, Palma-Durán SA, Gutiérrez-Coronado ML, Campa-Siqueiros MM, Grajeda-Cota P, Caire-Juvera G. Phytoestrogen Concentrations in Human Urine as Biomarkers for Dietary Phytoestrogen Intake in Mexican Women. Nutrients. 2017; 9:1078.
Article38. Ritchie MR, Morton MS, Deighton N, Blake A, Cummings JH. Plasma and urinary phyto-oestrogens as biomarkers of intake: validation by duplicate diet analysis. Br J Nutr. 2004; 91:447–457. PMID: 15005831.
Article39. Fraser GE, Jaceldo-Siegl K, Henning SM, Fan J, Knutsen SF, Haddad EH, Sabaté J, Beeson WL, Bennett H. Biomarkers of dietary intake are correlated with corresponding measures from repeated dietary recalls and food-frequency questionnaires in the Adventist Health Study-2. J Nutr. 2016; 146:586–594. PMID: 26843587.
Article40. Heald CL, Bolton-Smith C, Ritchie MR, Morton MS, Alexander FE. Phyto-oestrogen intake in Scottish men: use of serum to validate a self-administered food-frequency questionnaire in older men. Eur J Clin Nutr. 2006; 60:129–135. PMID: 16205743.
Article41. Tseng M, Olufade T, Kurzer MS, Wähälä K, Fang CY, van der Schouw YT, Daly MB. Food frequency questionnaires and overnight urines are valid indicators of daidzein and genistein intake in U.S. women relative to multiple 24-h urine samples. Nutr Cancer. 2008; 60:619–626. PMID: 18791925.
Article42. French MR, Thompson LU, Hawker GA. Validation of a phytoestrogen food frequency questionnaire with urinary concentrations of isoflavones and lignan metabolites in premenopausal women. J Am Coll Nutr. 2007; 26:76–82. PMID: 17353586.
Article43. Whitton C, Ho JC, Tay Z, Rebello SA, Lu Y, Ong CN, van Dam RM. Relative validity and reproducibility of a food frequency questionnaire for assessing dietary intakes in a multi-ethnic Asian population using 24-h dietary recalls and biomarkers. Nutrients. 2017; 9:1059.
Article44. Yamori Y, Sagara M, Arai Y, Kobayashi H, Kishimoto K, Matsuno I, Mori H, Mori M. Soy and fish as features of the Japanese diet and cardiovascular disease risks. PLoS One. 2017; 12:e0176039. PMID: 28430815.
Article45. Yu D, Shu XO, Li H, Yang G, Cai Q, Xiang YB, Ji BT, Franke AA, Gao YT, Zheng W, Zhang X. Dietary isoflavones, urinary isoflavonoids, and risk of ischemic stroke in women. Am J Clin Nutr. 2015; 102:680–686. PMID: 26245809.46. Ding M, Franke AA, Rosner BA, Giovannucci E, van Dam RM, Tworoger SS, Hu FB, Sun Q. Urinary isoflavonoids and risk of type 2 diabetes: a prospective investigation in US women. Br J Nutr. 2015; 114:1694–1701. PMID: 26370252.
Article47. Wu Y, Zhang L, Na R, Xu J, Xiong Z, Zhang N, Dai W, Jiang H, Ding Q. Plasma genistein and risk of prostate cancer in Chinese population. Int Urol Nephrol. 2015; 47:965–970. PMID: 25971353.
Article48. Michikawa T, Inoue M, Sawada N, Tanaka Y, Yamaji T, Iwasaki M, Shimazu T, Sasazuki S, Mizokami M, Tsugane S. The Japan Public Health Center-based Prospective Study Group. Plasma isoflavones and risk of primary liver cancer in Japanese women and men with hepatitis virus infection: a nested case-control study. Cancer Epidemiol Biomarkers Prev. 2015; 24:532–537. PMID: 25542831.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Estimated dietary isoflavone intake among Korean adults
- Survey on the Consumption of the Phytoestrogen Isoflavone in Postmenopausal Korean Women
- A Study on Dietary Isoflavone Intake from Soy Food and Urinary Isoflavone Excretion and, Menopausal Symptoms in Korean Women in Rural Areas
- Relation between Health Examination Outcome and Intake of Soy Food and Isoflavone among adult male in Seoul
- Effect of Soy Isoflavone Intake on Water Maze Performance and Brain Acetylcholinesterase Activity in Rats